Piracetam
Synonym(s):2-(2-Oxopyrrolidino)acetamide;2-Oxo-1-pyrrolidineacetamide
- CAS NO.:7491-74-9
- Empirical Formula: C6H10N2O2
- Molecular Weight: 142.16
- MDL number: MFCD00079246
- EINECS: 231-312-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-31 21:46:14
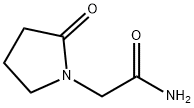
What is Piracetam?
Absorption
Piracetam displays a linear and time-dependent pharmacokinetic properties with low intersubject variability over a large range of doses. Piracetam is rapidly and extensively absorbed following oral administration with the peak plasma concentration is reached within 1 hour after dosing in fasted subjects. Following a single oral dose of 3.2 g piracetam, the peak plasma concentration (Cmax) was 84 μg/mL. Intake of food may decrease the Cmax by 17% and increase the time to reach Cmax (Tmax) from 1 to 1.5 hours. Tmax in the cerebrospinal fluid is achieved approximately 5 hours post-administration .
The absolute bioavailability of piracetam oral formulations is close to 100% and the steady state plasma concentrations are achieved within 3 days of dosing .
Toxicity
The cases of overdose with piracetam is rare. The highest reported overdose with piracetam was oral intake of 75g which was associated with diarrhea and abdominal pain; the signs were most likely related to the extreme high dose of sorbitol contained in the used formulation. In cases of acute, significant overdosage, stomach emptying by gastric lavage or induced emesis is recommended as there are no known antidotes for piracetam . Management for an overdose will most likely be symptomatic treatment and may include hemodialysis, where the extraction efficacy of the dialyser is 50 to 60% for the drug .
Oral LD50 in a mouse acute toxicity study was 2000 mg/kg .
Description
Piracetam is an unregistered cognition enhancer widely used to treat neurological disorders. It was sold as a dietary supplement until FDA ordered this practice to be discontinued in 2010. It remains available online and in some US stores. H. Morren reported the synthesis of piracetam in 1966. Earlier this year, D. Croker et al. reported?a method to monitor the crystallization of piracetam?to ensure that the desired stable polymorph is formed.
Description
Piracetam is a nootropic agent with an indication as adjunctive treatment for myoclonus of cortical origin, as well as tardive dyskinesia. However, piracetam is not approved by the FDA for any medical use in the USA. Piracetam should not be prescribed in patients with Huntington disease, those with serious kidney problems, or patients who have experienced a brain haemorrhage. Bleeding problems are a further caution to be taken into account. Although there is little evidence for piracetam’s efficacy in terms of long- term benefits for the treatment of mild cognitive impairments, recent studies showed that it is effective in the treatment of cognitive disorders of cerebrovascular and traumatic origins. With regard to piracetam’s behavioural profile, its beneficial effects on lowering depression and anxiety appear to be higher than its positive effects on memory.
Chemical properties
White crystalline powder. Odorless, slightly bitter taste. Soluble in water, slightly soluble in ethanol, almost insoluble in ether.
Originator
Nootropyl, UCB ,France,1972
The Uses of Piracetam
Piracetam has been used to study crystal structure and physicochemical properties of six co-crystals of piracetam. It has also been used to study its in vitro antioxidant properties.
The Uses of Piracetam
Piracetam is most commonly used for breath-holding attacks, seizure disorder (epilepsy), antinauseant, dizziness (vertigo), a learning disorder marked by difficulty reading (dyslexia), nootropic, cortical myoclonus, and a movement disorder often caused by antipsychotic drugs (tardive dyskinesia).
Background
Piracetam is a nootropic drug in the racetams group, with chemical name 2-oxo-1-pyrrolidine acetamide. It shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid and is a cyclic derivative of the neurotransmitter γ-aminobutyric acid (GABA). However its mechanism of action differ from that of endogenous GABA. Piracetam has neuroprotective and anticonvulsant properties and is reported to improve neural plasticity . Its efficacy is documented in cognitive disorders and dementia, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia although the clinical application in these conditions is not yet established. Piracetam has effects on the vascular system by reducing erythrocyte adhesion to the vascular endothelium, hindering vasospasms and facilitating microcirculation .
Originally marketed by UCB Pharma in 1971, piracetam was the first nootropic drug to modulate cognitive function without causing sedation or stimulation . It is not approved for any medical or dietary use by the FDA. In the UK, piracetam is prescribed mainly for myoclonus, but is used off-label for other conditions such as learning difficulties in children, memory loss or other cognitive defects in the elderly, and sickle-cell vaso-occlusive crises . Evidence to support its use for many conditions is unclear.
Indications
Indicated in adult patients suffering from myoclonus of cortical origin, irrespective of aetiology, and should be used in combination with other anti-myoclonic therapies .
What are the applications of Application
Piracetam is a modulator of neurotransmitter-induced ion flux
Definition
ChEBI: Piracetam is an organonitrogen compound and an organooxygen compound. It is functionally related to an alpha-amino acid. It is a compound suggested to be both a nootropic and a neuroprotective agent.
Preparation
Prepared by condensing 2-pyrrolidinone with ethyl chloroacetate in the presence of a metal hydride and then converting the ester into an amide with ammonia.
Manufacturing Process
2-Pyrrolidone is first reacted with sodium hydride, then with ethyl chloroacetate to give ethyl 2-oxo-1-pyrrolidine acetate.
A solution of 0.3 mol of ethyl 2-oxo-1-pyrrolidine acetate in 300 ml of methanol, saturated with ammonia at 20° to 30°C, is heated at 40° to 50°C for 5 hours, while continuously introducing ammonia. The reaction mixture is evaporated to dryness and the residue recrystallized from isopropanol. 2-Oxo1-pyrrolidineacetamide is obtained in a yield of 86%. MP 151.5° to 152.5°C.
Therapeutic Function
Psychotropic
Biological Activity
Nootropic that displays cognitive enhancing properties. Proposed to enhance neurotransmission via modulation of ion flux; potentiates Na + influx through AMPA receptors. Facilitates efficiency of cholinergic neurotransmission at muscarinic receptors.
Biochem/physiol Actions
Piracetam (2-oxo-1-pyrrolidinyl-acetamide) is a nootropic drug, which enhances memory and facilitates learning. Piracetam also acts as a vasodilator and improves blood flow. It has a potential to treat cognitive impairment in aging, brain injury, memory and balance problems. In addition, piracetam is also used to treat peripheral vascular disease.
Pharmacokinetics
Piracetam is known to mediate various pharmacodynamic actions:
Neuronal effects:
Piracetam modulates the cholinergic, serotonergic, noradrenergic, and glutamatergic neurotransmission although the drug does not display high affinity to any of the associated receptors (Ki >10μM). Instead, piracetam increases the density of postsynaptic receptors and/or restore the function of these receptors through stabilizing the membrane fluidity . In the forebrain of aging mice, the density of NMDA receptors was increased by approximately 20% following 14 days of piracetam treatment. Based on the findings of various animal and human studies, the cognitive processses including learning, memory, attention and consciousness were enhanced from piracetam therapy without inducing sedation and psychostimulant effects . Piracetam mediate neuroprotective effects against hypoxia-induced damage, intoxication, and electroconvulsive therapy .
In two studies involving alcohol-treated rats with evidences of withdrawal-related neuronal loss, piracetam was shown to reduce the extent of neuronal loss and increase the numbers of synapses in the hippocampus by up to 20% relative to alcohol-treated or alcohol-withdrawn rats . This suggests that piracetam is capable in promoting neuroplasticity when recoverable neural circuits are present . Although the mechanism of action is not fully understood, administration of piracetam prior to a convulsant stimulus reduces the seizure severity and enhances the anticonvulsant effectiveness of conventional antiepileptics such as carbamazepine and diazepam .
Vascular effects:
Piracetam is shown to increase the deformability of erythrocytes, reduce platelet aggregation in a dose-dependent manner, reduce the adhesion of erythrocytes to vascular endothelium and capillary vasospasm. In healthy volunteers, piracetam mediated a direct stimulant effect on prostacycline synthesis and reduced the plasma levels of fibrinogen and von Willebrand’s factors (VIII: C; VIII R: AG; VIII R: vW) by 30 to 40% . Potentiated microcirculation is thought to arise from a combination of effects on erythrocytes, blood vessels and blood coagulation .
Clinical Use
Myoclonus
Side Effects
Most common adverse effects are hyperkinesia, irritability, and weight gain; less commonly, patients can report asthenia, depression, and drowsiness.
Safety Profile
Mildly toxic by ingestion. Whenheated to decomposition it emits toxic fumes of NOx.
Metabolism
As large proportion of total piracetam administered is excreted as unchanged drug, there is no known major metabolism of piracetam .
Metabolism
The plasma half life of piracetam is approximately 5 hours following oral or intravenous administration. The half life in the cerebrospinal fluid was 8.5 hours. The apparent total body clearance is 80-90 mL/min.
Storage
Room temperature
Purification Methods
This typical nootropic (Alzheimer) drug modulates Na flux in AMPA receptors and is purified by recrystallisation from isoPrOH. [Gouilaev & Senning Brain Research Rev 19 180 1994.]
Mode of action
Piracetam is a nootropic drug in the racetams group, with chemical name 2-oxo-1-pyrrolidine acetamide. It shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid and is a cyclic derivative of the neurotransmitter γ-aminobutyric acid (GABA). However its mechanism of action differ from that of endogenous GABA. Piracetam has neuroprotective and anticonvulsant properties and is reported to improve neural plasticity. Its efficacy is documented in cognitive disorders and dementia, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia although the clinical application in these conditions is not yet established. Piracetam has effects on the vascular system by reducing erythrocyte adhesion to the vascular endothelium, hindering vasospasms and facilitating microcirculation.
Properties of Piracetam
| Melting point: | 151-152,5 C |
| Boiling point: | 259.72°C (rough estimate) |
| Density | 1.2298 (rough estimate) |
| refractive index | 1.5010 (estimate) |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water, soluble in ethanol (96 per cent). It shows polymorphism (5.9). |
| form | neat |
| pka | 15.67±0.40(Predicted) |
| form | Solid |
| color | Crystals from isopropanol |
| Water Solubility | Soluble in water (100mM) |
| InChI | InChI=1S/C6H10N2O2/c7-5(9)4-8-3-1-2-6(8)10/h1-4H2,(H2,7,9) |
| CAS DataBase Reference | 7491-74-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Piracetam(7491-74-9) |
Safety information for Piracetam
Computed Descriptors for Piracetam
| InChIKey | GMZVRMREEHBGGF-UHFFFAOYSA-N |
| SMILES | N1(CC(N)=O)CCCC1=O |
Piracetam manufacturer
New Products
BOC-L-4-HYDROXYPROLINE 2-bromo 6-methyl pyridine 2,3 Diamino pyridine 5-Iodo-2-(1-methylethyl)-3(2H)-pyridazinone 1-(difluoromethyl)-N-methylcyclobutan-1-amine Tert-Butyl N-[3-(dimethylcarbamoyl)prop-2-en-1-yl]carbamate 2-Fluoro-4-methoxy-6-methylbenzenamine Ethyl 1-[[1-[(1,1-dimethylethoxy)carbonyl]-3-azetidinyl]methyl]-1H-pyrazole-4-carboxylate 2-(4-Methyl-1,2,5-oxadiazol-3-yl)-1H-benzimidazole Trimethyl(phenylthio)silane Polycaprolactone(2000)-PEG(20000)-Polycaprolactone(2000) Diacrylate Ascorbyl Tetraisopalmitate or Tetrahexyldecyl Ascorbate PolyoxyethyleneOleylCetylEtherSulfosuccinate Diethylene Glycol Monoethyl Ether, Tween 20 or Polysorbate 20 Castor Oil, Ethoxylated, Cremophor EL or PEG-35 Castor Oil (S)-2-((tert-butyldiphenylsilyl)oxy)-1-((4S,5S)-2,2-dimethyl-5-(((methylsulfonyl)oxy)methyl)-1,3-dioxolan-4-yl)ethyl methanesulfonate methyl 2-bromo-6-(tert-butyl)nicotinate 2-Hydroxy-4-methylnicotinic acid methyl 5-amino-3-(1,1-dioxidotetrahydro-2H-1,2-thiazin-2-yl)-2-fluorobenzoate 3-(naphthalen-1-ylsulfonyl)-1H-indazol-5-amine 7-methoxy-8-(2-morpholinoethoxy)-4-((3,4,5-trimethoxyphenyl)amino)benzo[g]quinoline-3-carbonitrile Dimethylaluminum isopropoxide 3-(Dimethyl amino) Propyl Magnesium Chloride 1.0M in THFRelated products of tetrahydrofuran


You may like
-
 Piracetam 99%View Details
Piracetam 99%View Details -
 7491-74-9 95-99 %View Details
7491-74-9 95-99 %View Details
7491-74-9 -
 Piracetam 98% CAS 7491-74-9View Details
Piracetam 98% CAS 7491-74-9View Details
7491-74-9 -
 Piracetam Raw Material, Piracetam Powder API BP EP USP CAS 7491-74-9View Details
Piracetam Raw Material, Piracetam Powder API BP EP USP CAS 7491-74-9View Details
7491-74-9 -
 7491-74-9 Piracetam PowderView Details
7491-74-9 Piracetam PowderView Details
7491-74-9 -
 Piracetam Api PowderView Details
Piracetam Api PowderView Details
7491-74-9 -
 Piracetam Api PowderView Details
Piracetam Api PowderView Details
7491-74-9 -
 Piracetam Api PowderView Details
Piracetam Api PowderView Details
7491-74-9
