Paclitaxel
Synonym(s):Baccatin III N-benzyl-β-phenylisoserine Ester, TAXOL;O-De[(2R,3S)-3-(Benzoylamino)-2-hydroxy-3-phenylpropanoyl]paclitaxel;Paclitaxel;TAXOL InSolution, Baccatin III N-benzyl-β-phenylisoserine Ester
- CAS NO.:33069-62-4
- Empirical Formula: C47H51NO14
- Molecular Weight: 853.92
- MDL number: MFCD00869953
- EINECS: 608-826-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-10 16:01:40
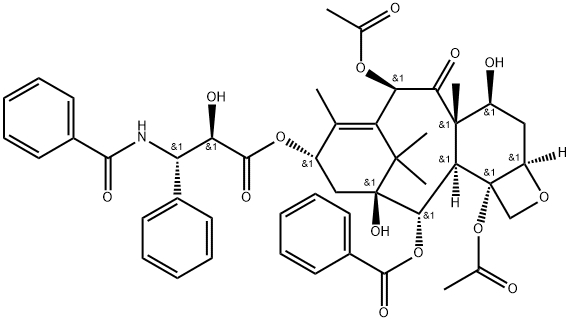
What is Paclitaxel?
Absorption
When a 24 hour infusion of 135 mg/m^2 is given to ovarian cancer patients, the maximum plasma concentration (Cmax) is 195 ng/mL, while the AUC is 6300 ng?h/mL.
Toxicity
Rat (ipr) LD50=32530 μg/kg. Symptoms of overdose include bone marrow suppression, peripheral neurotoxicity, and mucositis. Overdoses in pediatric patients may be associated with acute ethanol toxicity.
Description
Paclitaxel, a natural product isolated from the bark of the Pacific yew, is effective in treating refractory metastatic ovarian cancer. Unlike any other antineoplastic agents, paclitaxel appears to have several possible mechanisms of action, including an antimicrotubule action through the promotion of tubulin polymerization and stabilization of microtubules, thereby, halting mitosis and promoting cell death. The supply of paclitaxel is limited by its low natural abundance and currently it is being manufactured by a semi-synthetic route from deacetylbaccatin Ⅲ that is isolated from the needles of the yew tree. Recent completion of two total syntheses of taxol conquered the structural complexity of the title compound and may be useful in obtaining certain closely related analogs, some of which have been found to have antitumor activity. Paclitaxel has potential uses in the treatment of metastatic breast cancer, lung cancer, head and neck cancer, and malignant melanoma.
Description
Paclitaxel, commonly known by its trade name Taxol, is a chemotherapy drug that has been used to treat many types of cancer. Despite its several adverse side effects, it is on the World Health Organization''s List of Essential Medicines.
In 1971, as the sixth part of a series of articles on plant antitumor agents, Mansukh C. Wani and colleagues at the Research Triangle Institute (NC) and Duke University (Durham, NC) reported the isolation of a complex molecule from the bark of the Pacific yew tree (Taxus brevifolia) that they named “taxol”. In the article’s introduction, the authors state, “Taxol has potent antileukemic and tumor inhibitory properties and is the first compound possessing the taxane ring [that] has been demonstrated to have such activity.” The taxane “ring” is actually a tricyclic structure that forms the core of the molecule (see images).
Wani et al. provided very little information about “taxol’s” anticancer activity; the article focused on the chemistry and spectra of the compound and some of its derivatives. During the next two decades, Wani, his colleague Monroe E. Wall, and several other researchers, under the auspices of the National Cancer Institute (NCI), investigated the potential drug’s activity against various cancers. They began clinical trials in 1984. During the 1970s and ’80s, almost 10 t of Pacific yew bark were harvested and extracted for experimental work and the trials.
Finally, in 1989, NCI offered to turn the project over to a commercial pharmaceutical company. The offer generated surprisingly little interest; but by the end of the year, the technology was transferred to Bristol-Meyers Squibb (BMS)—the same year that the BMS merger was concluded. BMS obtained the generic name paclitaxel for the drug and registered the brand name Taxol with the US Office of Patents and Trademarks. The NCI–BMS deal and the trademarked name soon became the subject of much controversy.
BMS received its first drug approval for paclitaxel from the US Food and Drug Administration in 1992. As of 2018, FDA approvals include treatment of breast, pancreatic, ovarian, and non–small-cell lung cancers; and Kaposi''s sarcoma. Paclitaxel has been extremely thoroughly researched: As of this writing, SciFinder lists 73,164 references, including mentions in 68 United States and international patents.
The Uses of Paclitaxel
Paclitaxel is an antineoplastic that used to treat patients with lung, ovarian, breast cancer, head and neck cancer, and advanc ed forms of Kaposi's sarcoma. Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It is also used in the study of structure and function of microtubles into tubulin.
Background
Paclitaxel is a chemotherapeutic agent marketed under the brand name Taxol among others. Used as a treatment for various cancers, paclitaxel is a mitotic inhibitor that was first isolated in 1971 from the bark of the Pacific yew tree which contains endophytic fungi that synthesize paclitaxel. It is available as an intravenous solution for injection and the newer formulation contains albumin-bound paclitaxel marketed under the brand name Abraxane.
Indications
Used in the treatment of Kaposi's sarcoma and cancer of the lung, ovarian, and breast. Abraxane? is specfically indicated for the treatment of metastatic breast cancer and locally advanced or metastatic non-small cell lung cancer.
What are the applications of Application
Taxol is an antineoplastic and apoptosis inducing agent, also used to stabilize tubulin obtained from pig brain.
Pharmacokinetics
Paclitaxel is a taxoid antineoplastic agent indicated as first-line and subsequent therapy for the treatment of advanced carcinoma of the ovary, and other various cancers including breast cancer. Paclitaxel is a novel antimicrotubule agent that promotes the assembly of microtubules from tubulin dimers and stabilizes microtubules by preventing depolymerization. This stability results in the inhibition of the normal dynamic reorganization of the microtubule network that is essential for vital interphase and mitotic cellular functions. In addition, paclitaxel induces abnormal arrays or "bundles" of microtubules throughout the cell cycle and multiple asters of microtubules during mitosis.
Metabolism
Hepatic. In vitro studies with human liver microsomes and tissue slices showed that paclitaxel was metabolized primarily to 6a-hydrox-ypaclitaxel by the cytochrome P450 isozyme CYP2C8; and to two minor metabolites, 3’-p-hydroxypaclitaxel and 6a, 3’-p-dihydroxypaclitaxel, by CYP3A4.
Properties of Paclitaxel
| Melting point: | 213 °C (dec.)(lit.) |
| Boiling point: | 774.66°C (rough estimate) |
| Density | 0.200 |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | methanol: 50 mg/mL, clear, colorless |
| form | powder |
| appearance | white crystalline powder |
| color | white |
| Water Solubility | 0.3mg/L(37 ºC) |
Safety information for Paclitaxel
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H340:Germ cell mutagenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Paclitaxel
| InChIKey | RCINICONZNJXQF-MZXODVADSA-N |
Abamectin manufacturer
Jigs Chemical ltd
Green Vision Life Sciences Pvt Ltd.
Zyphars Biopharmaceuticals Pvt. Ltd
Basil Drugs AND Pharmaceuticals Pvt Ltd
Ralington Pharma
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran

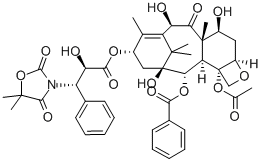
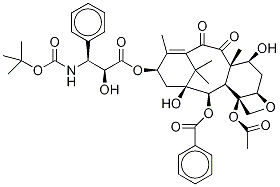
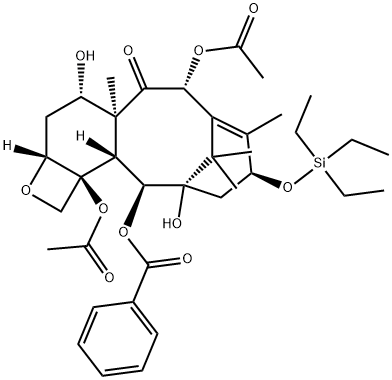
You may like
-
 33069-62-4 Paclitaxel 98%View Details
33069-62-4 Paclitaxel 98%View Details
33069-62-4 -
 Paclitaxel 99%View Details
Paclitaxel 99%View Details -
 Baccatin-III 99%View Details
Baccatin-III 99%View Details -
 Paclitaxel 33069-62-4 / 1203669-79-7 98%View Details
Paclitaxel 33069-62-4 / 1203669-79-7 98%View Details
33069-62-4 / 1203669-79-7 -
 33069-62-4 / 1203669-79-7 98%View Details
33069-62-4 / 1203669-79-7 98%View Details
33069-62-4 / 1203669-79-7 -
 Paclitaxel 98%View Details
Paclitaxel 98%View Details
33069-62-4 / 1203669-79-7 -
 33069-62-4 / 1203669-79-7 Paclitaxel 98%View Details
33069-62-4 / 1203669-79-7 Paclitaxel 98%View Details
33069-62-4 / 1203669-79-7 -
 33069-62-4 / 1203669-79-7 Paclitaxel 98%View Details
33069-62-4 / 1203669-79-7 Paclitaxel 98%View Details
33069-62-4 / 1203669-79-7