Nitrofurantoin
Synonym(s):N-(5-Nitro-2-furfurylidene)-1-aminohydantoin;Furadoxyl;Nitrofurantoine
- CAS NO.:67-20-9
- Empirical Formula: C8H6N4O5
- Molecular Weight: 238.16
- MDL number: MFCD00003224
- EINECS: 200-646-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-14 17:56:46
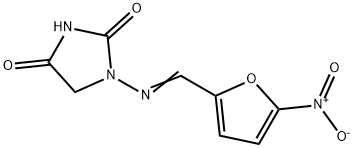
What is Nitrofurantoin?
Absorption
Nitrofurantoin reaches a Cmax of 0.875-0.963mg/L with an AUC of 2.21-2.42mg*h/L. It is 38.8-44.3% bioavailable. Taking nitrofurantoin with food increases the absorption and duration of therapeutic concentrations in the urine.
Toxicity
Symptoms of overdose include vomiting. In case of overdose, induce vomiting if it has not already occurred and increase fluid intake to promote urination. In extreme cases, nitrofurantoin can be removed from circulation by dialysis.
Description
Nitrofurantoin is an antibacterial medication used primarily to treat urinary tract and bladder infections. It has been sold under more than two dozen trade names, including Macrobid; but it is now almost always supplied as a generic.
The first US patent on the synthesis of nitrofurantoin was awarded in 1952 to Kenyon J. Hayes at the long-defunct Eaton Laboratories1 (Norwich, NY). The drug was introduced in 1953. In his 2015 book Basic Principles of Drug Discovery and Development, Benjamin E. Blass called nitrofurantoin “a surprisingly successful drug” because of its market longevity.
In 2018, a controversy erupted over the cost of the liquid formulation of nitrofurantoin. The only two US companies that produce the formulation, Nostrum Laboratories (Kansas City, MO) and Casper Pharma (East Brunswick, NJ), dramatically increased their prices: Nostrum, from the already expensive US$475 per bottle, to $2,393 and Casper to $2,800. Needless to say, an uproar ensued, heightening demands for pharma industry price reforms, an issue of much concern in the US Congress and elsewhere.
1. You can purchase vintage postcards of the Eaton buildings on Amazon and other online sellers.
The Uses of Nitrofurantoin
A nitrofuran antibiotic with low resistance potential that is rapidly metabolized by mammals. Active against both Gram-positive and Gram-negative bacteria. Nitrofurantoin is also a prooxidant that is cytotoxic due to the generation of intracellular H2O2. Antibacterial.
Indications
Nitrofurantoin is indicated to treat acute uncomplicated urinary tract infections.
Background
Nitrofurantoin is a nitrofuran antibiotic used to treat uncomplicated urinary tract infections. Nitrofurantoin is converted by bacterial nitroreductases to electrophilic intermediates which inhibit the citric acid cycle as well as synthesis of DNA, RNA, and protein. This drug is more resistant to the development of bacterial resistance because it acts on many targets at once. Nitrofurantoin is a second line treatment to trimethoprim/sulfamethoxazole.
Nitrofurantoin was granted FDA approval on 6 February 1953.
What are the applications of Application
Nitrofurantoin is a nitrofuran antibiotic
Pharmacokinetics
Nitrofurantoin interferes with vital processes in bacteria, which leads to their death. Nitrofurantoin rapidly reaches therapeutic concentrations in the urine and is also cleared rapidly.
Metabolism
0.8-1.8% of a dose is metabolized to aminofurantoin, and ≤0.9% of a dose is metabolized to other metabolites.
Properties of Nitrofurantoin
| Melting point: | 268°C |
| Boiling point: | 380.75°C (rough estimate) |
| Density | 1.5824 (rough estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMF: soluble50mg/mL |
| appearance | yellow to orange crystals or powder |
| form | crystalline |
| color | yellow |
| Water Solubility | <0.01 g/100 mL at 19 ºC |
| Sensitive | Light Sensitive & Hygroscopic |
Safety information for Nitrofurantoin
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Nitrofurantoin
Abamectin manufacturer
Prajna generics pvt ltd
SKVen Technologies Pvt.Ltd.
Salvavidas Pharmaceutical Pvt., Ltd.
Tooba Pharmaceuticals Private Limited
Srini Pharmaceuticals Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran






You may like
-
 NITROFURANTOIN 99%View Details
NITROFURANTOIN 99%View Details
67-20-9 -
 Nitrofurantoin 67-20-9 98%View Details
Nitrofurantoin 67-20-9 98%View Details
67-20-9 -
 Nitrofurantoin 98%View Details
Nitrofurantoin 98%View Details
67-20-9 -
 Nitrofurantoin 67-20-9 98%View Details
Nitrofurantoin 67-20-9 98%View Details
67-20-9 -
 67-20-9 98%View Details
67-20-9 98%View Details
67-20-9 -
 Nitrofurantoin 98%View Details
Nitrofurantoin 98%View Details
67-20-9 -
 Nitrofurantoin 98%View Details
Nitrofurantoin 98%View Details
67-20-9 -
 67-20-9 98%View Details
67-20-9 98%View Details
67-20-9