NITRIC OXIDE
Synonym(s):Nitrogen monoxide
- CAS NO.:10102-43-9
- Empirical Formula: NO*
- Molecular Weight: 30.01
- MDL number: MFCD00011525
- EINECS: 233-271-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-21 11:29:48
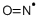
What is NITRIC OXIDE?
Absorption
Nitric oxide is absorbed systemically after inhalation.
Description
near room temperature (its liquid density at 20°C is 1.45 g/cm3). Nitrogen monoxide (NO) is commonly called nitric oxide,Nitric oxide is colorless and has a sharp sweet odor;Nitric oxide is nonfl ammable, toxic gases.Nitric oxide is a free radical that quickly reacts in air to produce nitrogen dioxide.It is also an important biological messenger and transmitter.
Description
Nitric oxide (NO) is a colorless gas and stable free radical that turns blue when it is liquefied or solidified. It was discovered and studied in 1772 by J. Priestley, who called it “nitrous air”. A toxic gas and air pollutant, NO has many industrial uses, especially in the production of nitric acid.
In the 1980s it was discovered that NO is the active metabolite released from nitroglycerine and amyl nitrite, vasodilators that are used for treating heart conditions such as angina. R. F. Furchgott, L. J. Ignarro, and F. Murad won the 1998 Nobel Prize in Physiology or Medicine for their work on NO’s role as a physiological signaling molecule. NO is released by the noni plant (Morinda citrifolia), which has been used as a “cure-all” by Pacific islanders for thousands of years.
The Uses of NITRIC OXIDE
Indications
For the treatment of term and near-term (>34 weeks) neonates with hypoxic respiratory failure
Background
Nitric oxide or Nitrogen monoxide is a chemical compound with chemical formula NO. This gas is an important signaling molecule in the body of mammals including humans and is an extremely important intermediate in the chemical industry. It is also a toxic air pollutant produced by automobile engines and power plants.
Nitric oxide (NO) should not be confused with nitrous oxide (N2O), a general anaesthetic, or with nitrogen dioxide (NO2) which is another poisonous air pollutant.
The nitric oxide molecule is a free radical, which is relevant to understanding its high reactivity. It reacts with the ozone in air to form nitrogen dioxide, signalled by the appearance of the reddish-brown color.
Pharmacokinetics
Persistent pulmonary hypertension of the newborn (PPHN) occurs as a primary developmental defect or as a condition secondary to other diseases such as meconium aspiration syndrome (MAS), pneumonia, sepsis, hyaline membrane disease, congenital diaphragmatic hernia (CDH), and pulmonary hypoplasia. In these states, pulmonary vascular resistance (PVR) is high, which results in hypoxemia secondary to right-to-left shunting of blood through the patent ductus arteriosus and foramen ovale. In neonates with PPHN, Nitric oxide improves oxygenation (as indicated by significant increases in PaO2). Nitric oxide appears to increase the partial pressure of arterial oxygen (PaO2) by dilating pulmonary vessels in better entilated areas of the lung, redistributing pulmonary blood flow away from lung regions with low ventilation/perfusion (V/Q) ratios toward regions with normal ratios.
Metabolism
via pulmonary capillary bed
Properties of NITRIC OXIDE
| Melting point: | −163.6 °C(lit.) |
| Boiling point: | −151.7 °C(lit.) |
| Density | d-150.2 (liq) 1.27; Relative d (gas) 1.036 (air = 1); Absolute d (gas) 1.227 (air = 1) |
| solubility | At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 21 volumes of water. |
| form | colorless gas |
| Water Solubility | slightly soluble H2O [HAW93] |
Safety information for NITRIC OXIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Gas Cylinder Compressed Gases GHS04  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H270:Oxidising gases H280:Gases under pressure H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for NITRIC OXIDE
| InChIKey | MWUXSHHQAYIFBG-UHFFFAOYSA-N |
Abamectin manufacturer
Vadilal Chemicals Limited
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE) 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THFRelated products of tetrahydrofuran

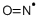






You may like
-
 10102-43-9 Nitric oxide 98%View Details
10102-43-9 Nitric oxide 98%View Details
10102-43-9 -
 2,3 Dichloro-4-Hydroxy Aniline 39183-17-0 99%View Details
2,3 Dichloro-4-Hydroxy Aniline 39183-17-0 99%View Details
39183-17-0 -
 17673-56-2 99%View Details
17673-56-2 99%View Details
17673-56-2 -
 13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 -
 143-07-7 99%View Details
143-07-7 99%View Details
143-07-7 -
 Ethanolamine 141-43-5 99%View Details
Ethanolamine 141-43-5 99%View Details
141-43-5 -
 616-47-7 99%View Details
616-47-7 99%View Details
616-47-7 -
 20776-67-4 99%View Details
20776-67-4 99%View Details
20776-67-4