Malonic acid
Synonym(s):13C Labeled malonic acid;Propanedioic acid;Propanedioic acid-13C3
- CAS NO.:141-82-2
- Empirical Formula: C3H4O4
- Molecular Weight: 104.06
- MDL number: MFCD00002707
- EINECS: 205-503-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-27 15:57:40
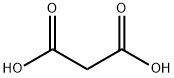
What is Malonic acid?
Description
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word (malon) meaning 'apple'.
Description
Malonic acid, formally propanedioic acid, is the second-smallest aliphatic dicarboxylic acid. (Oxalic acid?is the smallest.) It should not be confused with malic or maleic acid, both of which also contain two carboxyls.
Malonic acid is a white crystalline solid with a decomposition point of ≈135 °C. It is highly soluble in water and oxygenated solvents. It has numerous commercial uses: It is a precursor to specialty polyesters; it is used in the manufacture of barbiturates, coatings, and biodegradable containers; and it is even a component of surgical adhesives.
French chemist Victor Dessaignes reported the first synthesis of malonic acid in 1858; he made it by oxidatively decomposing four-carbon malic acid with potassium dichromate. Since then, it has been synthesized commercially starting from chloroacetic acid, diethyl malonate, and even sodium acetate.
In the past two decades, much work has been done on biobased syntheses of malonic acid. Most recently, the Berkeley, CA, venture-capital startup Lygos raised US$13 million to scale up a process that uses bioengineered yeast strains to?produce malonic acid from sugars.
Lygos, by the way, was reported by Pliny the Elder as a former name of the “town of Byzantium”. Today, of course, it’s the city of Istanbul.
The Uses of Malonic acid
Malonic acid is used as an intermediate in the manufacture of barbiturates and other pharmaceuticals. It is a component used as a stabilizer in many high-end cosmetic and pharmaceutical products. Malonic acid is also used as building block in chemical synthesis, specifically to introduce the molecular group -CH2-COOH. It is used for the introduction of an acetic acid moiety under mild conditions by Knoevenagel condensation and subsequent decarboxylation.
Properties of Malonic acid
| Melting point: | 132-135 °C (dec.) (lit.) |
| Boiling point: | 140℃(decomposition) |
| Density | 1.619 g/cm3 at 25 °C |
| Flash point: | 157°C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 1 M NaOH: soluble100mg/mL, clear to slightly hazy, colorless to faintly yellow |
| form | Liquid |
| color | White |
| Water Solubility | 1400 g/L (20 ºC) |
Safety information for Malonic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Malonic acid
| InChIKey | OFOBLEOULBTSOW-UHFFFAOYSA-N |
Abamectin manufacturer
ANJI BIOSCIENCES
JSK Chemicals
S D Fine Chem Limited
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine N-Benzyl-3-hydroxypiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran
You may like
-
 141-82-2 Malonic acid(R-3) 98%View Details
141-82-2 Malonic acid(R-3) 98%View Details
141-82-2 -
 141-82-2 98%View Details
141-82-2 98%View Details
141-82-2 -
 Malonic acid, 99% 99%View Details
Malonic acid, 99% 99%View Details
141-82-2 -
 Malonic acid 141-82-2 99%View Details
Malonic acid 141-82-2 99%View Details
141-82-2 -
 141-86-6 98%View Details
141-86-6 98%View Details
141-86-6 -
 4-Amino-2-Chloropyridine 14432-12-3 98%View Details
4-Amino-2-Chloropyridine 14432-12-3 98%View Details
14432-12-3 -
 13466-41-6 98%View Details
13466-41-6 98%View Details
13466-41-6 -
 Ammonium HexachloropalladateView Details
Ammonium HexachloropalladateView Details
19168-23-1