Hydrogen peroxide
Synonym(s):Catalase Test;Hydrogen Dioxide;Hydrogen peroxide solution;Hydrogenii peroxidum 30 per centum;Perhydrol
- CAS NO.:7722-84-1
- Empirical Formula: H2O2
- Molecular Weight: 34.01
- MDL number: MFCD00011333
- EINECS: 231-765-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-15 13:03:24
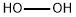
What is Hydrogen peroxide?
Absorption
It is reported that hydrogen peroxide is decomposed before absorption in the intestine. Solutions of hydrogen peroxide displays poor penetration when applied to tissue .
Toxicity
Oral LD50 in mouse is 2000 mg/kg, and dermal LD50 is 4060 mg/kg in rat and 2000 mg/kg pig. LC50 of hydrogen peroxide vapours in rat is 2000 mg/m at 4 hours .
Oral ingestion of high dose hydrogen peroxide may cause chest and stomach pain, loss of consciousness, motor disorders, microhemorrhages and moderate leucocytosis in humans. Inhalation of highly concentrated vapours causes extreme irritation of nose and throat .
Hydrogen peroxide has no known carcinogenic potential. It was shown to be mutagenic to bacteria (Salmonella typhimurium) and the fungi, Neurospora crassa and Aspergillis chevallieri, and induced DNA damage in Escheria coli . It also caused sister chromatid exchanges and chromosomal aberrations in mammalian cells in vitro .
Description
Hydrogen peroxide, found in medicine cabinets around the world, is a powerful oxidizing agent. On human skin, an enzyme unlocks the oxidizing potential allowing it to kill germs. Concentrated hydrogen peroxide is used as a reactant in glow sticks, color safe bleach, and even rocket fuels.
Description
Hydrogen peroxide is the simplest peroxide and is considered a strong oxidizer. It exists as a colorless liquid and is miscible with H2O in all proportions. For laboratory purposes 30% Hydrogen peroxide (in H2O) is most common.
The Uses of Hydrogen peroxide
hydrogen peroxide is a bleaching and oxidizing agent, detergent, and antiseptic. It is generally recognized as a safe preservative, germ killer, and skin bleacher in cosmetics.
The Uses of Hydrogen peroxide

A mixture of the SM (1.7 g, 3.3 mmol), AcOH (15 mL), Na2WO4 (209 mg, 0.60 mmol), H2O2 (50%, 2.3 mL, 6.6 mmol) was stirred at RT overnight. The solvent was removed in vacuo and ice/H2O was added to the residue. The mixture was extracted with DCM. The combined organics were dried, concentrated, and purified by silica gel chromatography to provide the product. [1.65 g, 90%]
The Uses of Hydrogen peroxide
Hydrogen peroxide has a number of environmental uses. These include water treatment, odorcontrol, oxidation of pollutants, and corrosion control. Hydrogen peroxide is used to removeiron, manganese, and hydrogen sulfide from water supplies and wastewater. The oxidation ofsubstances such as hydrogen sulfide reduces odors. Because H2O2 decomposes into oxygen andwater, it has the added advantage of lowering the biological oxygen demand of wastewater.ese include water treatment, odorcontrol, oxidation of pollutants, and corrosion control. Hydrogen peroxide is used to removeiron, manganese, and hydrogen sulfide from water supplies and wastewater. The oxidation of substances such as hydrogen sulfide reduces odors. Because H2O2 decomposes into oxygen andwater, it has the added advantage of lowering the biological oxygen demand of wastewater.
Indications
Indicated to be used as a disinfectant and sterilizer.
Background
Hydrogen peroxide is the simplest peroxide with a chemical formula H2O2. Hydrogen peroxide is an unstable compound in the presence of a base or catalyst, and is typically stored with a stabilizer in a weakly acidic solution. If heated to its boiling point, it may undergo potentially explosive thermal decomposition. Hydrogen peroxide is formed in the body of mammals during reduction of oxygen either directly in a two-electron transfer reaction . As a natural product of metabolism, it readily undergoes decomposition by catalase in normal cells .
Due to its potent and broad-spectrum antimicrobial actions, hydrogen peroxide is used in both liquid and gas form for preservative, disinfection and sterilization applications as an oxidative biocide . It is used in industrial and cosmetic applications as a bleaching agent. Hydrogen peroxide is also considered as a generally recognized as safe compound by the FDA ; it is used as an antimicrobial agent in starch and cheese products, and as an oxidizing and reducing agent in products containing dried eggs, dried egg whites, and dried egg yolks.
Preparation
Hydrogen peroxide is commercially produced by autooxidation of ethyl anthraquinol in a solvent such as toluene or ethylbenzene. The product ethyl anthraquinone is reduced by hydrogen over supported nickel or platinum catalyst to regenerate back the starting material, ethyl anthraquinol for a continuous production of H2O2. The reaction steps are:

Hydrogen peroxide may also be made by heating 2-propanol with oxygen at 100°C under 10 to 20 atm pressure:
(CH3)2CHOH (CH3)2C(OH)OOH → CH3COCH3 + H2O2
Vapor phase partial oxidation of hydrocarbons also yield H2O2. However, several by-products are generated, the separations of which make the process difficult and uneconomical.
Hydrogen peroxide may also be prepared by treating barium peroxide with dilute sulfuric acid:
BaO2 + 2H2SO4 → H2O2 + BaSO4
Another preparative method involves electrolytic conversion of aqueous sulfuric acid to peroxydisulfate followed by hydrolysis to H2O2 (Weissenstein process). The reaction steps are as follows:
2H2SO4 → H2S2O8 + H2
H2SO5 + H2O → H2SO4 + H2SO5
H2SO5 + H2O → H2O2 + H2SO4
An earlier method, which currently is no longer practiced commercially, involved oxidation of phenyl hydrazine:
 Hydrogen peroxide obtained this way may contain many impurities, depending on the process used. Such impurities are removed by ion exchange, solvent extraction, and distillation. Dilute solutions of H2O2 may be purified
Hydrogen peroxide obtained this way may contain many impurities, depending on the process used. Such impurities are removed by ion exchange, solvent extraction, and distillation. Dilute solutions of H2O2 may be purified
and concentrated by fractional distillation at reduced pressures.
Definition
hydrogen peroxide: A colourlessor pale blue viscous unstable liquid,H2O2; r.d. 1.44; m.p. –0.41°C; b.p.150.2°C. As with water, there is considerablehydrogen bonding in theliquid, which has a high dielectricconstant. It can be made in the laboratoryby adding dilute acid to bariumperoxide at 0°C. Large quantitiesare made commercially by electrolysisof KHSO4.H2SO4 solutions. Anotherindustrial process involvescatalytic oxidation (using nickel, palladium,or platinum with an anthraquinone)of hydrogen and waterin the presence of oxygen. Hydrogenperoxide readily decomposes in lightor in the presence of metal ions togive water and oxygen. It is usuallysupplied in solutions designated byvolume strength. For example, 20-volume hydrogen peroxide wouldyield 20 volumes of oxygen per volumeof solution. Although the peroxidesare formally salts of H2O2, thecompound is essentially neutral.Thus, the acidity constant of the ionizationH2O2 + H2O ?H3O+ + HO2–is 1.5 × 10-12 mol dm-3. It is a strongoxidizing agent, hence its use as amild antiseptic and as a bleachingagent for cloth, hair, etc. It has alsobeen used as an oxidant in rocketfuels.
Production Methods
Hydrogen peroxide is currently produced on a large scale using the anthraquinone autooxidation procedure, which was developed in the 1940s. In this process, an anthraquinone, typically 2-ethyl-anthraquinone, is hydrogenated to a hydroquinone (2-ethyl-anthrahydroquinone) then reoxidized back to the anthraquinone (2-ethyl-anthraquinone) while forming hydrogen peroxide . A metal palladium or nickel catalyst is used to convert the anthraquinone to the hydroquinone, followed by autooxidation in air to generate hydrogen peroxide. The anthraquinone and hydrogen peroxide are separated; the former is recycled to repeat the process while the hydrogen peroxide is purified.
Reactions
Hydrogen peroxide reacts with many compounds, such as borates, carbonates, pyrophosphates, sulfates, silicates, and a variety of organic carboxylic acids, esters, and anhydrides to give peroxy compounds or peroxyhydrates. A number of these compounds are stable solids that hydrolyze readily to give hydrogen peroxide in solution.
Hazard
Hydrogen peroxide is a strong oxidizing agent. Concentrated solutions, even a 30% aqueous solution, should be handled carefully. The compound decomposes violently in the presence of trace impurities. Inhibitors are, therefore, added at trace levels to prevent decomposition.
Health Hazard
Concentrated solutions of hydrogen peroxide are very caustic and can cause burns of skin and mucous membranes. Exposure to its vapors can produce body irritation, lacrimation, sneezing, and bleaching of hair. A dose of 500 mg/kg by dermal route caused convulsions and deaths in rabbits. The oral LD50 value for 90% peroxide solution in mice is 2000 mg/kg.
Oral administration of hydrogen peroxide produced tumors in gastrointestinal tract in mice. There is limited evidence of carcinogenicity in animals. Cancercausing effects of hydrogen peroxide in humans are unknown. Padma and coworkers (1989) reported the promoting effect of hydrogen peroxide on tobacco-specific Nnitrosoamines in inducing tumors in the lung, liver, stomach, and cheek pouch in Syrian golden hamsters and mice. The incidence of cheek pouch tumors increased when peroxide was administered concurrently or applied for a long period after a low initiator dose of N-nitrosamines. .
Pharmacokinetics
Hydrogen peroxide exhibits antimicrobial properties against most forms of microorganisms, including dormant forms with known high resistance profiles, such as bacterial spores and protozoal cysts. It acts as an oxidative biocide to generate free radical species to induce DNA, protein and membrane lipid damage via oxidation.
Toxicology
Acute toxicities (LD50) of hydrogen peroxide for rats are 700 mg/kg/b.w. and 21 mg/kg/b.w. by subcutaneous injection and intravenous injection, respectively. When large amounts of hydrogen peroxide were injected directly into the stomachs of rats, weight and blood protein concentrations were changed slightly. When hydrogen peroxide was mixed with feed, however, no abnormalities were observed. The use of bactericides has been limited due to their toxicity to humans, and only hydrogen peroxide currently is recognized for use.
Potential Exposure
PotentialExposure:CompoundDescription: Drug,Tumorigen,Mutagen, Human Data; Hormone, PrimaryIrritant (90%); Mutagen, Human Data (20%). Hydrogenperoxide is used in the manufacture of acetone, antichlor,antiseptics, benzoyl peroxide, buttons, disinfectants, phar-maceuticals, felt hats, plastic foam, rocket fuel, sponge rub-ber, and pesticides; as a food and feed additive; flavor; as apackaging material; in bleaching bone; feathers, flour, fruit,fur, gelatin, glue, hair, ivory, silk, soap, straw, textiles, wax,and wood pulp; and as an oxygen source in respiratory pr0-tective equipment. Other specific occupations with potentialexposure include liquor and wine agers, dyers, electropla-ters, fat refiners, photographic film developers, wool prin-ters, veterinarians, and water treaters.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from ex posure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24- -48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmo-nary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Metabolism
Hydrogen peroxide is reduced by glutathione peroxidase, which is an endogenous enzyme in human tissue. It is rapidly decomposed to oxygen and water when in contact with catalase, an enzyme found in blood and most tissues .
Shipping
Hydrogen peroxide, stabilized, or hydrogen per-oxide aqueous solutions, stabilized with > 60% hydrogenperoxide,requirea shipping label of “OXIDIZER,CORROSIVE." It falls in Hazard Class 5.1 and PackingGroup I.Hydrogen peroxide, aqueous solutions with > > 40% but not>60% hydrogen peroxide (stabilized as necessary), requiresa shipping label of“OXIDIZER, CORROSIVE." It falls inHazard Class 5.1 and Packing Group II.Hydrogen peroxide, aqueous solutions with not < 20% butnot >40% hydrogen peroxide (stabilized as necessary), requires a shipping label of“OXIDIZER, CORROSIVE."" Itfalls in Hazard Class 5.1 and Packing Group II.Hydrogen peroxide, aqueous solutions with not < <8% but<20% hydrogen peroxide (stabilized as necessary), requiresa shipping label of“OXIDIZER" It falls in Hazard Class5.1 and Packing Group III.
Incompatibilities
Hydrogen peroxide reacts with certain organic functional groups (ethers, acetals, etc.) to form peroxides, which may explode upon concentration. Reaction with acetone generates explosive cyclic dimeric and trimeric peroxides. Explosions may also occur on exposure of hydrogen peroxide to metals such as sodium, potassium, magnesium, copper, iron, and nickel.
Properties of Hydrogen peroxide
| Melting point: | -33 °C |
| Boiling point: | 108 °C |
| Density | 1.13 g/mL at 20 °C |
| vapor density | 1.1 (vs air) |
| vapor pressure | 23.3 mm Hg ( 30 °C) |
| refractive index | 1.3350 |
| Flash point: | 107°C |
| storage temp. | 10-30°C |
| solubility | diethyl ether: soluble |
| form | Solution |
| appearance | Colorless liquid |
| pka | 11.5(at 25℃) |
| Specific Gravity | approximate 1.13 |
| color | ≤10(APHA) |
| Odor | Slightly pungent, irritating odor |
| PH | 2-4 (H2O, 20°C) |
| PH Range | 6 - 8 at 25 °C |
| Water Solubility | miscible |
| Merck | 14,4798 |
| BRN | 3587191 |
| Exposure limits | TLV-TWA 1 ppm (~1.5 mg/m3) (ACGIH), MSHA,andOSHA),IDLH75 ppm(NIOSH). |
| Dielectric constant | 84.2(0℃) |
| Stability: | Slightly unstable - will very slowly decompose. Decomposition is promoted by catalysts and heating, so store cool. Light sensitive, keep in the dark. May contain stabilizer. Reacts with rust, brass, zinc, nickel, finely powdered metals, copper and iron and their alloys. |
| CAS DataBase Reference | 7722-84-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydrogen peroxide(7722-84-1) |
| IARC | 3 (Vol. 36, Sup 7, 71) 1999 |
| EPA Substance Registry System | Hydrogen peroxide (7722-84-1) |
Safety information for Hydrogen peroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Hydrogen peroxide
| InChIKey | MHAJPDPJQMAIIY-UHFFFAOYSA-N |
Hydrogen peroxide manufacturer
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran



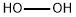
You may like
-
 Peroxide CASView Details
Peroxide CASView Details -
 Hydrogen Peroxide CASView Details
Hydrogen Peroxide CASView Details -
 Hydrogen Peroxide CASView Details
Hydrogen Peroxide CASView Details -
 Hydrogen Peroxide H2o2, 99%, 25LView Details
Hydrogen Peroxide H2o2, 99%, 25LView Details
7722-84-1 -
 Hydrogen Peroxide 50%View Details
Hydrogen Peroxide 50%View Details
7722-84-1 -
 Liquid Hydrogen Peroxide, 35%View Details
Liquid Hydrogen Peroxide, 35%View Details
7722-84-1 -
 Industrial Grade Liquid Hydrogen Peroxide, 35%, 200 L DrumView Details
Industrial Grade Liquid Hydrogen Peroxide, 35%, 200 L DrumView Details
7722-84-1 -
 Hydrogen Peroxide 50%View Details
Hydrogen Peroxide 50%View Details
7722-84-1
