Hydrogen bromide
Synonym(s):HBr;Hydrobromic acid;Hydrogen bromide in acetic acid;Hydrogen Bromide Solution, Hydrobromic Acid
- CAS NO.:10035-10-6
- Empirical Formula: BrH
- Molecular Weight: 80.91
- MDL number: MFCD00011323
- EINECS: 233-113-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-01 18:08:31
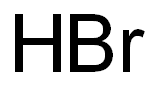
What is Hydrogen bromide?
Description
Hydrobromic Acid is a strong acid formed by dissolving the
diatomic molecule HBr in water. “Constant-boiling”
hydrobromic acid is an aqueous solution that distills at
124.3°C and contains 47.6% HBr by weight. Hydrobromic
acid has a pKa of 9, making it a stronger acid
than hydrochloric acid, but not as strong as HI, hydroiodic
acid. Hydrobromic acid is one of the strongest
mineral acids known.
Hydrobromic acid is mainly used for the production
of inorganic bromides, especially the bromides of zinc,
calcium, and sodium. It is a useful reagent for generating
organobromine compounds. Certain ethers are
cleaved with HBr. It also catalyzes alkylation reactions
and the extraction of certain ores. Industrially significant
organic compounds prepared from hydrobromic
acid include allyl bromide, tetrabromobis(phenol),
and bromoacetic acid.
Hydrobromic acid can be prepared in the laboratory
via the reaction of Br2, SO2 and water. Another laboratory
preparation involves the production of anhydrous
HBr, which is then dissolved in water.
Hydrobromic acid has commonly been prepared
industrially by reacting bromine with either sulfur or
phosphorous and water. However, it can also be
produced electrolytically. It can also be prepared by
treating bromides with nonoxidizing acids like phosphoric
or acetic acids. Hydrobromic acid is available
commercially in various concentrations and purities.
Description
Hydrobromic acid (HBr) is commonly sold as a 48% (by weight) solution in water. HBr works well as a Lewis acid catalyst but the increased nucleophilicity of bromide relative to chloride generally makes hydrochloric acid the preferable choice. However, in certain reactions such as in the deprotection of methyl ethers, the increased nucleophilicity of bromide is advantageous. HBr is also a reasonable choice in reactions where the presence of bromide already exists, such as Sandmeyer reactions and brominations.
The Uses of Hydrogen bromide
Hydrogen bromide is used as a reagent and
catalyst in several types of organic reactions
such as the formation of alkyl bromides from
alcohols.
It is also used as a source material in the
preparation of inorganic bromides. Hydrogen
bromide serves as a catalyst in alkylation reactions.
It has also been reportedly used in the
controlled oxidation of aliphatic and alicyclic
hydrocarbons to peroxides, ketones, and acids.
In organic synthesis, hydrogen bromide is used
to substitute bromine for aliphatic chlorine in
the presence of aluminum catalyst.
The Uses of Hydrogen bromide

A mixture of the SM (2 g, 5.82 mmol), HBr in AcOH (12 mL), and aq HBr (6 mL) was heated at 100 C for 3 h. The reaction mixture was cooled to RT, extracted with EtOAc (2 x 100 mL), washed with brine, dried (Na2SO4), and concentrated to provide the product as an off-white solid. [1.3 g]
Properties of Hydrogen bromide
| Melting point: | −87 °C(lit.) |
| Boiling point: | −67 °C(lit.) |
| Density | 1.49 g/mL at 25 °C (lit.) |
| Flash point: | 40°C |
| storage temp. | Store below +30°C. |
| solubility | soluble |
| appearance | Colorless to light yellow liquid (48% w/w aq.) |
| form | Solution |
| color | Light yellow, brown |
| Odor | Sharp, irritating odor detectable at 2 ppm |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
Safety information for Hydrogen bromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Hydrogen bromide
Abamectin manufacturer
Bhakti Impex
PACIFIC ORGANICS PVT LTD
ANJI BIOSCIENCES
Sagar Speciality Chemicals Private Limited
JSK Chemicals
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE) 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THFRelated products of tetrahydrofuran


You may like
-
 Hydrobromic Acid 48% 10035-10-6 99%View Details
Hydrobromic Acid 48% 10035-10-6 99%View Details
10035-10-6 -
 Hydrobromic Acid 48 % in water 10035-10-6 99%View Details
Hydrobromic Acid 48 % in water 10035-10-6 99%View Details
10035-10-6 -
 Hydrobromic acid 48% 10035-10-6 98%View Details
Hydrobromic acid 48% 10035-10-6 98%View Details
10035-10-6 -
 Hydrobromic acid in acetic acid 10035-10-6 98%View Details
Hydrobromic acid in acetic acid 10035-10-6 98%View Details
10035-10-6 -
 10035-10-6 Hydrobromic Acid 48% 98%View Details
10035-10-6 Hydrobromic Acid 48% 98%View Details
10035-10-6 -
 10035-10-6 99%View Details
10035-10-6 99%View Details
10035-10-6 -
 143-07-7 99%View Details
143-07-7 99%View Details
143-07-7 -
 20776-67-4 99%View Details
20776-67-4 99%View Details
20776-67-4