Gefitinib
Synonym(s):N-(3-Chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine;ZD1839
- CAS NO.:184475-35-2
- Empirical Formula: C22H24ClFN4O3
- Molecular Weight: 446.9
- MDL number: MFCD04307832
- EINECS: 643-034-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-22 11:00:39
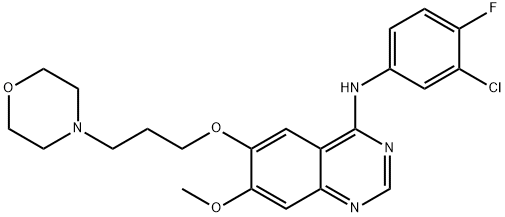
What is Gefitinib?
Absorption
Absorbed slowly after oral administration with a mean bioavailability of 60%. Peak plasma levels occurs 3-7 hours post-administration. Food does not affect the bioavailability of gefitinib.
Toxicity
The acute toxicity of gefitinib up to 500 mg in clinical studies has been low. In non-clinical studies, a single dose of 12,000 mg/m2 (about 80 times the recommended clinical dose on a mg/m2 basis) was lethal to rats. Half this dose caused no mortality in mice. Symptoms of overdose include diarrhea and skin rash.
Description
Gefitinib was introduced in Japan as a daily oral monotherapy for the treatment of inoperable or recurrent non-small cell lung cancers (NSCLC). This anilinoquinazoline derivative can be synthesized in 6 steps starting from 6,7-dimethoxyquinazolin-4(3H)-one by successive monodemethylationlacetylation of the 6-hydroxy-group followed by chlorination and reaction with 3-chloro-4-fluoroaniline, finally deacetylation and alkylation with 3-(4-morpholinyl)propylbromide complete the synthesis. Gefitinib reversibly inhibits the activity of the epidermal growth factor receptor tyrosine kinase (EGRF TK). This inhibits autophosphorylation of EGRF and blocks the cascade of intracellular events which have been implicated in the proliferation, survival and metastasis of cancer cells. Gefitinib diplays good selectivity for the EGRF TK relative to other growth factors in human umbilical endothelial cells. It is similarly selective relative to other kinases, for example cerB2. Data from two large phase II studies in patients with pretreated NSCLC have shown that gefitinib induces a response rate approaching 20% in patients receiving the agent as a second line therapy and approximately 10% in those pretreated with more lines of chemotherapy. Gefitinib has good bioavailability and is metabolized in the liver via the cytochrome P450 3A4 enzyme system with a mean elimination half life of 28 h. Gefitinib has been generally well tolerated in cancer patients with predominant side effects being acne-like skin-rash, diarrhea, nausea, vomiting and mild to moderate myelosuppression. .
The Uses of Gefitinib
Gefitinib (Iressa, ZD-1839) is an EGFR inhibitor for Tyr1173, Tyr992, Tyr1173 and Tyr992 in the NR6wtEGFR and NR6W cells with IC50 of 37 nM, 37nM, 26 nM and 57 nM, respectively.
Background
Gefitinib (originally coded ZD1839) is a drug used in the treatment of certain types of cancer. Acting in a similar manner to erlotinib (marketed as Tarceva), gefitinib selectively targets the mutant proteins in malignant cells. It is marketed by AstraZeneca under the trade name Iressa.
Indications
For the continued treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of either platinum-based or docetaxel chemotherapies.
What are the applications of Application
Gefitinib is an EGFR-tyrosine kinase inhibitor
Pharmacokinetics
Gefitinib inhibits the intracellular phosphorylation of numerous tyrosine kinases associated with transmembrane cell surface receptors, including the tyrosine kinases associated with the epidermal growth factor receptor (EGFR-TK). EGFR is expressed on the cell surface of many normal cells and cancer cells.
Metabolism
Primarily hepatic via CYP3A4. Three sites of biotransformation have been identified: metabolism of the N-propoxymorpholino-group, demethylation of the methoxy-substituent on the quinazoline, and oxidative defluorination of the halogenated phenyl group.
Properties of Gefitinib
| Melting point: | 119-1200C |
| Boiling point: | 586.8±50.0 °C(Predicted) |
| Density | 1.322±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | Soluble in DMSO (up to 40 mg/ml) or in Ethanol (up to 4 mg/ml). |
| form | powder |
| color | white to beige |
Safety information for Gefitinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Gefitinib
Abamectin manufacturer
SUJALAM CHEMICALS
Zyphars Biopharmaceuticals Pvt. Ltd
Basil Drugs AND Pharmaceuticals Pvt Ltd
NATCO Pharma Limited
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE) 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THFRelated products of tetrahydrofuran

You may like
-
 184475-35-2 GEFITINIB BP -2 KG 99%View Details
184475-35-2 GEFITINIB BP -2 KG 99%View Details
184475-35-2 -
 Gefitinib 97%View Details
Gefitinib 97%View Details -
 Gefitinib 99%View Details
Gefitinib 99%View Details -
 184475-35-2 Gefitinib 98%View Details
184475-35-2 Gefitinib 98%View Details
184475-35-2 -
 184475-35-2 99%View Details
184475-35-2 99%View Details
184475-35-2 -
 Gefitinib 98%View Details
Gefitinib 98%View Details
184475-35-2 -
 184475-35-2 98%View Details
184475-35-2 98%View Details
184475-35-2 -
 Gefitinib 184475-35-2 99%View Details
Gefitinib 184475-35-2 99%View Details
184475-35-2