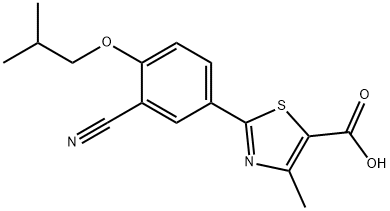
Febuxostat
Synonym(s):2-(3-Cyano-4-isobutoxyphenyl)-4-methyl-1,3-thiazole-5-carboxylic acid;Adenuric;Atenuri;Uloric
- CAS NO.:144060-53-7
- Empirical Formula: C16H16N2O3S
- Molecular Weight: 316.37
- MDL number: MFCD00871598
- EINECS: 682-158-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-13 18:00:15
What is Febuxostat?
Absorption
After oral administration, about 85% of febuxostat is absorbed rapidly. Tmax ranges from 1 to 1.5 hours. Following once-daily oral administration, Cmax was approximately 1.6 ± 0.6 mcg/mL at a dose of 40 mg febuxostat and 2.6 ± 1.7 mcg/mL at a dose of 80 mg febuxostat.
A high-fat meal decreased Cmax by 49% and AUC by 18%, but there were no clinically significant changes in the ability of febuxostat to decrease serum uric acid concentrations.
Toxicity
Oral lowest published toxic dose (TDLO) in humans is 1.82 mg/kg/14D (intermittent). Oral LD50 is 300 mg/kg in mice, 3200 mg/kg in rabbits, and 980 mg/kg in rats.
No dose-limiting toxicities were observed with febuxostat administered at doses up to 300 mg daily for seven days in healthy subjects. There are no reports of overdose of febuxostat in clinical studies and there is no known antidote. Overdose should be managed by symptomatic and supportive care.
Description
Febuxostat, a selective xanthine oxidase inhibitor, was launched for the chronic management of hyperuricemia in patients with
gout. Hyperuricemia is defined as a serum uric acid concentration
exceeding the limit of solubility. It predisposes affected persons to
gout, a disease characterized by the formation of crystals of monosodium urate or uric acid from supersaturated fluids in joints and other
tissues. Crystal deposition is asymptomatic, but it is revealed by bouts
of joint inflammation. If left untreated, further crystals accumulate in
joints and can form deposits known as tophi. A major aim in gout
management is the long-term reduction of serum uric acid concentrations below saturation levels, as this results in crystal dissolution and
eventual disappearance.
Febuxostat is a nonpurine derivative with higher potency and selectivity than allopurinol for inhibiting xanthine oxidase. It completely inhibits human xanthine oxidase
activity in the lung cancer cell line A549, whereas the activities of other enzymes involved in purine or pyrimidine metabolism (e.g., purine
nucleoside phosphorylase, adenosine deaminase, and pyrimidine
nucleoside phosphorylase) are affected by <4%.
The incidence of adverse events such as dizziness,
diarrhea, headache, and nausea with febuxostat was similar to allopurinol.
Febuxostat is contraindicated in patients being treated with the
xanthine oxidase substrates such as azathioprine, mercaptopurine, and
theophylline. Febuxostat can be synthesized in a multistep sequence
from 2,4-dicyanophenol, starting with the alkylation of the phenolic
hydroxyl group with isobutyl bromide and potassium carbonate, followed
by treatment with thioacetamide in hot dimethyl formamide to
yield 3-cyano-4-isobutoxythiobenzamide. Cyclization of the thioamide group with 2-chloroacetoacetic acid ethyl ester in refluxing ethanol
affords 2-(3-cyano-4-isoutoxyphenyl)-4-methylthiazole-5-carboxylic acid
ethyl ester, which is hydrolyzed with sodium hydroxide to produce
febuxostat.
The Uses of Febuxostat
Xanthine oxidase/xanthine dehydrogenase inhibitor. Used for treatment of hyperuricemia and chronic gout. 40-120 mg/day febuxostat was proven effective in lowering serum urate levels when administered to manage hyperuricemia in patients with gout.
Background
Febuxostat is a non-purine xanthine oxidase (XO) inhibitor. In early 2008, febuxostat was granted marketing authorization by the European Commission for the treatment of chronic hyperuricemia and gout. In the following year, the FDA for approved febuxostat for use in the chronic management of hyperuricemia in adult patients with gout who have an inadequate response or intolerance to allopurinol. Gout is a form of arthritis that is caused by the accumulation of uric acid crystal in or around a joint, leading to inflammation and further deposition of uric acid crystal deposition in bones, joints, tissues, and other organs in the long term. Gout is closely associated with hyperuricemia. Febuxostat works by inhibiting the activity of an enzyme that is responsible for the synthesis of uric acid, thereby reducing serum uric acid levels.
In February 2019, a black box warning for febuxostat was added, based on the findings of a post-market clinical study (the CARES trial) where there was an increased risk of cardiovascular (CV) fatal outcomes in patients with gout and known cardiovascular disease treated with febuxostat, when compared to those treated with allopurinol. The manufacturer and the FDA advise health professionals to limit the use of febuxostat to second-line therapy in patients who have inadequate response or intolerance to allopurinol, and to avoid the use of febuxostat in patients with cardiovascular diseases.
Indications
Febuxostat is indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable. It is not recommended for the treatment of asymptomatic hyperuricemia or secondary hyperuricemia.
What are the applications of Application
Febuxostat is an inhibitor xanthine oxidase and xanthine dehydrogenase
Pharmacokinetics
Febuxostat is a novel, selective xanthine oxidase/dehydrogenase inhibitor that works by decreasing serum uric acid in a dose-dependent manner. In healthy subjects, febuxostat decreased the mean serum uric acid and serum xanthine concentrations, as well as the total urinary uric acid excretion. Febuxostat at daily doses of 40-80 mg reduced the 24-hour mean serum uric acid concentrations by 40 to 55%. Closely related to the drug-induced reduction of serum uric acid levels and mobilization of urate crystals in tissue deposits, febuxostat is associated with gout flares.
Unlike allopurinol and oxypurinol, febuxostat has no inhibitory actions against other enzymes involved in purine and pyrimidine synthesis and metabolism, because it does not structurally resemble purines or pyrimidines.
Metabolism
Febuxostat is metabolized in the liver by UDP-glucuronosyltransferase (UGT) and Cytochrome P450 (CYP) enzymes, with the relative contribution of each enzyme isoform in the metabolism of febuxostat not fully elucidated. UGT1A1, UGT1A3, UGT1A9, and UGT2B7 mediate conjugation of febuxostat, which approximately accounts for 22–44% of the metabolism of the total dose administered, to produce the acyl-glucuronide metabolite. CYP1A2, CYP2C8, CYP2C9, and non-P450 enzymes are responsible for the oxidation reaction, which accounts for 2-8% of the metabolism of the dose. Oxidation reaction produces 67M-1, 67M-2, and 67M-4, which are pharmacologically active metabolites. 67M-1, 67M-2, and 67M-4 can further undergo glucuronidation and sulfation. Hydroxy metabolites are present in human plasma at much lower concentrations than the parent drug.
Properties of Febuxostat
| Melting point: | 238-239°(dec.) |
| Boiling point: | 536.6±60.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | powder |
| color | White to Off-White |
Safety information for Febuxostat
Computed Descriptors for Febuxostat
Abamectin manufacturer
Jigs Chemical ltd
GLP Pharma Standards
Cadila Pharmaceuticals Ltd
HEMA PHARMACEUTICALS PVT LTD
Siddhivinayakchemicals
Vadivarhe Speciality Chemicals Limited
KARPSCHEM LABORATORIES PVT. LTD.
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol 3-NITRO-5-ACETYL IMINODIBENZYL (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE) 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THFRelated products of tetrahydrofuran

You may like
-
 Febuxostat 99%View Details
Febuxostat 99%View Details -
 Febuxostat 98%View Details
Febuxostat 98%View Details -
 Febuxostat 98%View Details
Febuxostat 98%View Details -
 Febuxostat 99%View Details
Febuxostat 99%View Details
144060-53-7 -
 144060-53-7 Febuxostat 99%View Details
144060-53-7 Febuxostat 99%View Details
144060-53-7 -
 Febuxostat 144060-53-7 98%View Details
Febuxostat 144060-53-7 98%View Details
144060-53-7 -
 Febuxostat 99%View Details
Febuxostat 99%View Details
144060-53-7 -
 Febuxostat 98%View Details
Febuxostat 98%View Details
144060-53-7