Everolimus
- CAS NO.:159351-69-6
- Empirical Formula: C53H83NO14
- Molecular Weight: 958.22
- MDL number: MFCD00929329
- EINECS: 621-003-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-10 13:09:10
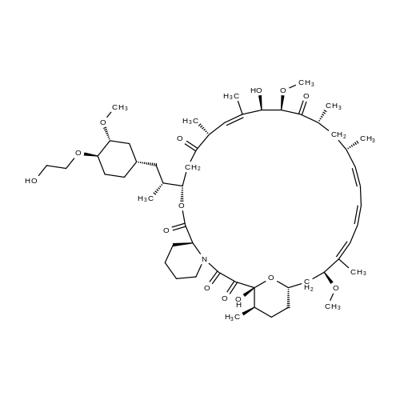
What is Everolimus?
Absorption
In patients with advanced solid tumors, peak everolimus concentrations are reached 1 to 2 hours after administration of oral doses ranging from 5 mg to 70 mg. Following single doses, Cmax is dose-proportional between 5 mg and 10 mg. At doses of 20 mg and higher, the increase in Cmax is less than dose-proportional, however AUC shows dose-proportionality over the 5 mg to 70 mg dose range. Steady-state was achieved within 2 weeks following once-daily dosing. Dose Proportionality in Patients with SEGA (subependymal giant-cell astrocytomas) and TSC (tuberous sclerosis complex): In patients with SEGA and TSC, everolimus Cmin was approximately dose-proportional within the dose range from 1.35 mg/m2 to 14.4 mg/m2.
Toxicity
IC50 of 0.63 nM.
Description
Everolimus, an oral immunosuppressant for the treatment of kidney and heart transplant rejection, is the 40-O-(2-hydroxyethyl) derivative of rapamycin. It has immunosuppressive properties similar to those of rapamycin, but with improved pharmacokinetic profile. Everolimus, like rapamycin, is a proliferation signal inhibitor that exerts its immunosuppressive effect by inhibiting the activation of p70 S6 kinase, thereby blocking growth factor-driven proliferation of T cells, B cells and vascular smooth muscle cells, and arresting cell cycle at the G1 phase. Inhibition of p70 S6 kinase activation by everolimus and rapamycin is mediated by their binding to FKBP12 (FK506 binding-protein 12). Everolimus inhibits FK506 binding to FKBP12 with an IC50 of 1.8–2.6 nM, and it is about 3- to 5-fold less potent than rapamycin (IC50=0.4–0.9 nM). The in vitro immunosuppressive activity of everolimus is also slightly less than that of rapamycin as demonstrated in a mixed lymphocyte reaction (MLR) assay (IC50=0.2–1.6 nM versus 0.07–0.5 nM, respectively) and in antigen-specific human helper T-cell clones (IC50=0.05–0.17nM versus 0.014–0.37nM, respectively). However, the in vivo immunosuppressive activity of oral everolimus 1–5 mg/ kg/day is similar to that of rapamycin at equivalent doses in rat models of renal or cardiac transplantation, localized graft-versus-host disease, and autoimmune glomerulonephritis. The recommended dosage of everolimus is 0.75 mg twice daily, and it is used in combination with cyclosporine microemulsion and corticosteroids.
The Uses of Everolimus
Everolimus (IX) (SDZ-RAD), was developed by Novartis as an immunosuppressant to be used in conjunction with cyclosporin in transplantation allograft rejection and was recently approved in the US in 2003. Another natural product that had been approved for use in transplantation is rapamycin (sirolimus) as an inejectable agent. In an attempt to develop an orally bioavailable immunosuppressant agent, many companies attempted modification of rapamycin itself.
Indications
Everolimus is indicated for the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane, after failure of treatment with letrozole or anastrozole. Indicated for the treatment of adult patients with progressive neuroendocrine tumors of pancreatic origin (PNET) with unresectable, locally advanced or metastatic disease. Indicated for the treatment of adult patients with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib. Indicated for the treatment of adult patients with renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery. Indicated in pediatric and adult patients with tuberous sclerosis complex (TSC) for the treatment of subependymal giant cell astrocytoma (SEGA) that requires therapeutic intervention but cannot be curatively resected.
Background
Everolimus is a derivative of Rapamycin (sirolimus), and works similarly to Rapamycin as an mTOR (mammalian target of rapamycin) inhibitor. It is currently used as an immunosuppressant to prevent rejection of organ transplants. In a similar fashion to other mTOR inhibitors Everolimus' effect is solely on the mTORC1 protein and not on the mTORC2 protein.
What are the applications of Application
Everolimus is an immunosuppresive inhibitor of FRAP (mTOR)
Metabolism
Everolimus is a substrate of CYP3A4 and PgP (phosphoglycolate phosphatase). Three monohydroxylated metabolites, two hydrolytic ring-opened products, and a phosphatidylcholine conjugate of everolimus were the 6 primary metabolites detected in human blood. In vitro, everolimus competitively inhibited the metabolism of CYP3A4 and was a mixed inhibitor of the CYP2D6 substrate dextromethorphan.
Properties of Everolimus
| Melting point: | NA |
| Boiling point: | 998.7±75.0 °C(Predicted) |
| Density | 1.18±0.1 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 100 mg/ml) or in Ethanol (up to 100 mg/ml). |
| form | solid |
| color | White |
| Water Solubility | Soluble in dimethysulfoxide,ethanol and chloroform. Slightly soluble in water. |
Safety information for Everolimus
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Everolimus
| InChIKey | HKVAMNSJSFKALM-GKUWKFKPSA-N |
Abamectin manufacturer
Humble Healthcare Limited
Ralington Pharma
BDR Pharmaceuticals International Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol 3-NITRO-5-ACETYL IMINODIBENZYL (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran


You may like
-
 Everolimus 98%View Details
Everolimus 98%View Details -
 159351-69-6 98%View Details
159351-69-6 98%View Details
159351-69-6 -
 Everolimus 159351-69-6 99%View Details
Everolimus 159351-69-6 99%View Details
159351-69-6 -
 159351-69-6 Everolimus 98%View Details
159351-69-6 Everolimus 98%View Details
159351-69-6 -
 159351-69-6 98%View Details
159351-69-6 98%View Details
159351-69-6 -
 Everolimus 98%View Details
Everolimus 98%View Details
159351-69-6 -
 159351-69-6 Everolimus 98%View Details
159351-69-6 Everolimus 98%View Details
159351-69-6 -
 Everolimus 99%View Details
Everolimus 99%View Details