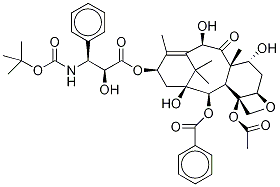
Docetaxel
Synonym(s):Docetaxel;Docetaxel trihydrate
- CAS NO.:114977-28-5
- Empirical Formula: C43H53NO14
- Molecular Weight: 807.88
- MDL number: MFCD00871399
- EINECS: 601-339-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-25 19:13:46
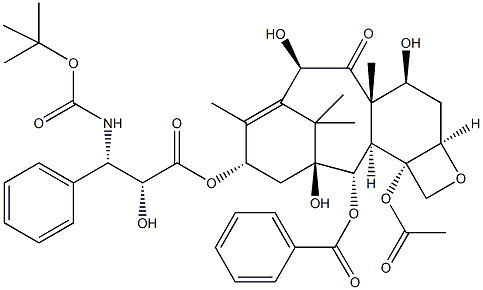
What is Docetaxel?
Absorption
The pharmacokinetic profile of docetaxel is consistent with a three-compartment model. The initial rapid decline represents the distribution to the peripheral compartments, and the late (terminal) phase is partly due to a relatively slow efflux of docetaxel from the peripheral compartment. The area under the curve (AUC) was dose proportional at doses between 70 mg/m2 and 115 mg/m2 with infusion times of 1 to 2 hours. In a group of patients with solid tumors given 100 mg/m2 of docetaxel intravenously, the Cmax and AUC were 2.41 μg/mL and 5.93 μg?h/mL, respectively.
Toxicity
There is no known antidote for an overdose of docetaxel injection. In case of overdose, patients should be closely monitored in specialized units. Some of the anticipated complications of overdosage include: bone marrow suppression, peripheral neurotoxicity, and mucositis. After an overdose is discovered, patients should receive granulocyte colony-stimulating factor (G-CSF) as soon as possible. Other appropriate symptomatic measures should be taken as needed.
In two reports of overdose, one patient received 150 mg/m2, and the other received 200 mg/m2 as 1-hour infusions. Both patients experienced severe neutropenia, mild asthenia, cutaneous reactions, and mild paresthesia, and recovered without incident. In rats, the oral LD50 of docetaxel is >2000 mg/kg. The intravenous LD50 in mice is 138 mg/kg.
Description
Docetaxel, a semi-synthetic member of the taxoid family, was introduced to the market in 1995, initially in South Africa and subsequently in various other markets, primarily for treating ovarian, breast, and non-small cell lung cancers. Similar to the naturally occurring antitumor agent paclitaxel, which was the first taxoid to be marketed, docetaxel facilitates tubulin assembly into stable microtubules and impedes their depolymerization, acting as a mitotic spindle poison and inducing a mitotic block in proliferating cells. This mechanism distinguishes taxoids from other classes of anticancer agents. Docetaxel has demonstrated twice the potency of paclitaxel in numerous in vitro assays and exhibits heightened cytotoxicity. Ongoing clinical trials are exploring its efficacy in treating other types of tumors, including pancreatic, gastric, head and neck cancers, and soft tissue sarcomas.
The Uses of Docetaxel
Docetaxel is a semisynthetic analog of taxol that inhibits microtubule disassembly (IC50 = 0.2 μM) and inhibits cell replication (IC50 = 0.13 μM). It has proven more effective than taxol in preventing the proliferation of cancer cells. Docetaxel has applications in breast cancer and hormone-refractory prostate cancer. This product is intended for research applications.
Indications
Docetaxel is indicated as a single agent for the treatment of locally advanced or metastatic breast cancer after chemotherapy failure; and with doxorubicin and cyclophosphamide as adjuvant treatment of operable node-positive BC. It is also indicated as a single agent for locally advanced or metastatic non-small cell lung cancer (NSCLC) after platinum therapy failure; and with cisplatin for unresectable, locally advanced or metastatic untreated NSCLC. For the treatment of metastatic castration-resistant prostate cancer, docetaxel is indicated with prednisone. Docetaxel is also indicated with cisplatin and fluorouracil for untreated, advanced gastric adenocarcinoma, including the gastroesophageal junction, and with cisplatin and fluorouracil for induction treatment of locally advanced squamous cell carcinoma of the head and neck (SCCHN).
Background
Docetaxel, a clinically established anti-mitotic chemotherapy medication, is utilized for treating various cancers, including breast, ovarian, and non-small cell lung cancer. As a complex diterpenoid molecule and a semisynthetic analogue of paclitaxel, docetaxel binds reversibly to microtubulin with high affinity in a 1:1 stoichiometric ratio. This interaction inhibits cell division and promotes cell death. Unlike paclitaxel, docetaxel demonstrates twice the potency as an inhibitor of microtubule depolymerization. Notably, it binds to microtubules but does not interact with dimeric tubulin. Despite its efficacy, the use of docetaxel may lead to undesired outcomes, including hepatic impairment, hematologic effects, enterocolitis, neutropenic colitis, hypersensitivity reactions, fluid retention, second primary malignancies, embryo-fetal toxicity, and tumor lysis syndrome. Approved by the FDA in 1996, docetaxel is available in solution for injection for intravenous or parenteral administration.
Pharmacokinetics
Docetaxel, classified as a taxoid antineoplastic agent, functions by facilitating the assembly of microtubules from tubulin dimers and stabilizing them, thus preventing depolymerization. This stabilization disrupts the normal dynamic reorganization of the microtubule network crucial for vital interphase and mitotic cellular functions. Moreover, docetaxel induces the formation of abnormal microtubule arrays or "bundles" throughout the cell cycle and multiple asters of microtubules during mitosis. However, the use of docetaxel may pose risks, including treatment-related deaths in patients with breast cancer and non-small cell lung cancer, hepatic impairment, hematologic effects, enterocolitis, and neutropenic colitis. Additional potential adverse effects include hypersensitivity reactions, fluid retention, the development of second primary malignancies, cutaneous reactions, neurologic reactions, eye disorders, asthenia, embryo-fetal toxicity, and tumor lysis syndrome.
Metabolism
Docetaxel undergoes hepatic metabolism. In vitro drug interaction studies revealed that docetaxel is metabolized by the CYP3A4 isoenzyme. CYP3A5 also plays a role in the metabolism of this drug. In humans, docetaxel is metabolized by CYP3A4/5 into four metabolites: M1, M2, M3 and M4. Docetaxel undergoes hydroxylation of the synthetic isobutoxy side chain, forming metabolite M2. The oxidation of M2 forms an unstable aldehyde that is immediately cyclised into the stereoisomers M1 and M3. M4 is then formed by the oxidation of M1/M3.
Properties of Docetaxel
| Melting point: | 186-192 °C (dec.) |
| Boiling point: | 900.5±65.0 °C(Predicted) |
| Density | 1.38 |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Practically insoluble in water, freely soluble in anhydrous ethanol, soluble in methylene chloride. |
| form | neat |
| color | White |
| Water Solubility | Soluble in dimethyl sulfoxide and ethanol. Insoluble in water. |
Safety information for Docetaxel
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity H362:Reproductive toxicity, effects on or via lactation H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Docetaxel
| InChIKey | BEDLLNJKXXVTSX-LWWLJZAUSA-N |
Abamectin manufacturer
AVD pharmaceuticals Pvt Ltd
Green Vision Life Sciences Pvt Ltd.
Zyphars Biopharmaceuticals Pvt. Ltd
Basil Drugs AND Pharmaceuticals Pvt Ltd
Kekule Pharma Limited
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran
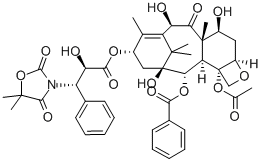
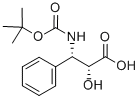
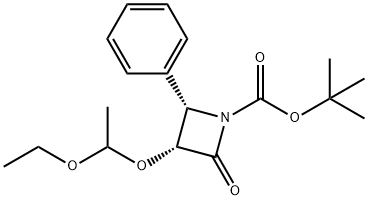
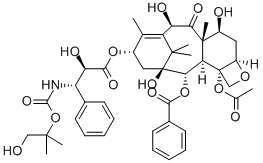
You may like
-
 Docetaxel 99%View Details
Docetaxel 99%View Details -
 114977-28-5 Docetaxel 98%View Details
114977-28-5 Docetaxel 98%View Details
114977-28-5 -
 Docetaxel 98%View Details
Docetaxel 98%View Details
114977-28-5 -
 114977-28-5 Docetaxel 98%View Details
114977-28-5 Docetaxel 98%View Details
114977-28-5 -
 114977-28-5 98%View Details
114977-28-5 98%View Details
114977-28-5 -
 114977-28-5 98%View Details
114977-28-5 98%View Details
114977-28-5 -
 Docetaxel 114977-28-5 98%View Details
Docetaxel 114977-28-5 98%View Details
114977-28-5 -
 Docetaxel 99%View Details
Docetaxel 99%View Details