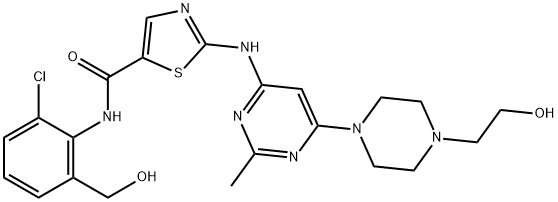
Dasatinib
Synonym(s):BMS 354825;BMS354825;BMS-354825;N-(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide
- CAS NO.:302962-49-8
- Empirical Formula: C22H26ClN7O2S
- Molecular Weight: 488.01
- MDL number: MFCD11046566
- EINECS: 801-607-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-21 10:16:54

What is Dasatinib?
Absorption
Dasatinib has a dose-proportional pharmacokinetic profile and a linear elimination between 15 mg/day (0.15 times the lowest approved recommended dose) and 240 mg/day (1.7 times the highest approved recommended dose). At 100 mg once a day, dasatinib has a Cmax and AUC of 82.2 ng/mL and 397 ng/mL*hr, respectively. In healthy adult subjects given dasatinib as dispersed tablets in juice, the adjusted geometric mean ratio compared to intact tablets was 0.97 for Cmax, and 0.84 for AUC. The Tmax of dasatinib is between 0.5 and 6 hours following oral administration. Following a single dose of 100 mg, a high-fat meal increases the AUC of dasatinib by 14%.
Toxicity
Overdose cases with dasatinib occurred in isolated cases during clinical studies. Patients that received 280 mg of dasatinib per day for 1 week developed severe myelosuppression and bleeding. Since dasatinib is associated with severe myelosuppression, patients that ingest more than the recommended dosage should be monitored closely for myelosuppression and receive appropriate supportive treatment.
Acute overdose in animals was associated with cardiotoxicity. In rodents, ventricular necrosis and valvular/ventricular/atrial hemorrhage were detected at single doses higher than or equal to 100 mg/kg (600 mg/m2). In monkeys receiving single doses higher than or equal to 10 mg/kg (120 mg/m2), there was a tendency for increased systolic and diastolic blood pressure. In rats, the oral LD50 of dasatinib is 50-100 mg/kg, and in monkeys, it is 25-45 mg/kg.
Description
Dasatinib, developed and marketed by Bristol Myers, is the first approved oral tyrosine kinase inhibitor which binds to multiple conformations of ABL kinase for the treatment of two leukemia indications: chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Dasatinib is a highly potent, ATPcompetitive ATPcompetitive kinase inhibitor which, at nanomolar concentrations, inhibits BCR-ABL, SRC family, c-KIT, EPHA2 and PDGFR-B.
The Uses of Dasatinib
Dasatinib is a novel, potent and multi-targeted inhibitor that targets Abl, Src and c-Kit, with IC50 of <1 nM, 0.8 nM and 79 nM, respectively.
Background
Dasatinib is an orally available multikinase inhibitor indicated for the treatment of Philadelphia chromosome (Ph)-positive leukemias. Ph is a chromosomal abnormality found in patients with chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL), where the ABL tyrosine kinase and the breakpoint cluster region (BCR) gene transcribe the chimeric protein BCR-ABL. BCR-ABL is associated with the uncontrolled activity of the ABL tyrosine kinase and is involved in the pathogenesis of CML and 15-30% of ALL cases. Dasatinib also inhibits a spectrum of kinases involved in cancer, including several SRC-family kinases.
Unlike imatinib, another tyrosine kinase used for the treatment of CML and Ph-positive ALL, dasatinib inhibits the active and inactive conformations of the ABL kinase domain. Also, mutations in the kinase domain of BCR-ABL may lead to relapse during imatinib treatment. Since dasatinib does not interact with some of the residues involved in those mutations, the use of this drug represents a therapeutic alternative for patients with cancers that have developed imatinib-resistance. The use of dasatinib was first approved by the FDA in 2006.
Indications
Dasatinib is indicated for the treatment of newly diagnosed adults with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase, as well as adults with chronic, accelerated, or myeloid or lymphoid blast phase Ph+ CML with resistance or intolerance to prior therapy including imatinib, and adults with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) with resistance or intolerance to prior therapy. Dasatinib is also indicated for the treatment of pediatric patients 1 year of age and older with Ph+ CML in chronic phase or newly diagnosed Ph+ ALL in combination with chemotherapy.
What are the applications of Application
Dasatinib is potent inhibitor, effective at sub-nanomolar concentrations, of the non-receptor tyrosine kinases Abl and Src as well as other members of the Src family.
Pharmacokinetics
Dasatinib is an orally available small-molecule multikinase inhibitor. During clinical trials, less than 1% of patients treated with dasatinib had QTc prolongation as an adverse reaction, and 1% experienced a QTcF higher than 500 ms. The use of dasatinib is also associated with myelosuppression, bleeding-related events, fluid retention, cardiovascular toxicity, pulmonary arterial hypertension, severe dermatologic reactions, tumor lysis syndrome and hepatotoxicity. It may also cause embryo-fetal toxicity and lead to adverse reactions associated with bone growth and development in pediatric patients.
Metabolism
In humans, dasatinib is mainly metabolized by CYP3A4, although flavin-containing monooxygenase 3 (FMO3) and uridine diphosphate-glucuronosyltransferase (UGT) enzymes are also involved in the formation of dasatinib metabolites. Five pharmacologically active dasatinib metabolites have been identified: M4, M5, M6, M20 and M24. M4, M20, and M24 are mainly generated by CYP3A4, M5 is generated by FMO3, and M6 is generated by a cytosolic oxidoreductase. M4 is equipotent to dasatinib and represents approximately 5% of the AUC. However, it is unlikely to play a major role in the observed pharmacology of dasatinib. M5 and M6 are more than 10 times less active than dasatinib and are considered minor circulating metabolites.
Properties of Dasatinib
| Melting point: | 275-286°C |
| Density | 1.408±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to 200 mg/ml). |
| form | White powder. |
| color | White or off-white |
Safety information for Dasatinib
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Dasatinib
Abamectin manufacturer
Jigs Chemical ltd
AVD pharmaceuticals Pvt Ltd
Bulat Pharmaceutical Pvt Ltd
Shilpa Medicare Limited (SML)
Vannsh Life Sciences Pvt Ltd
Basil Drugs AND Pharmaceuticals Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran



You may like
-
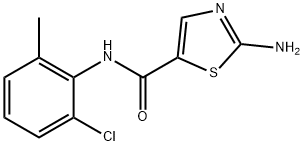
 Dasatinib 99%View Details
Dasatinib 99%View Details -
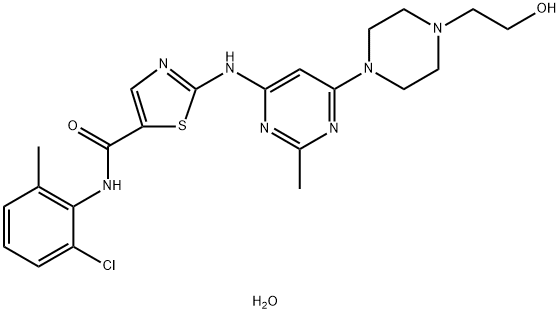
 302962-49-8 98%View Details
302962-49-8 98%View Details
302962-49-8 -
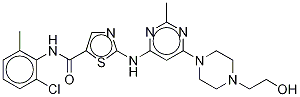
 Dasatinib 98%View Details
Dasatinib 98%View Details
302962-49-8 -
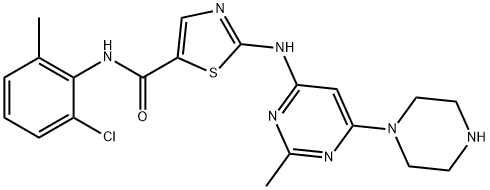
 302962-49-8 99%View Details
302962-49-8 99%View Details
302962-49-8 -
 Dasatinib 98%View Details
Dasatinib 98%View Details
302962-49-8 -
 Dasatinib 98%View Details
Dasatinib 98%View Details
302962-49-8 -
 Dasatinib 98%View Details
Dasatinib 98%View Details
302962-49-8 -
 Dasatinib 99%View Details
Dasatinib 99%View Details