D-Cycloserine
Synonym(s):(R)-4-Amino-3-isoxazolidone;D -Cycloserine;4-Amino-3-isoxazolidinone
- CAS NO.:68-41-7
- Empirical Formula: C3H6N2O2
- Molecular Weight: 102.09
- MDL number: MFCD00005353
- EINECS: 200-688-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-26 17:21:34
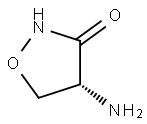
What is D-Cycloserine?
Absorption
Rapidly and almost completely absorbed (70 to 90%) from the gastrointestinal tract following oral administration.
Toxicity
Oral LD50 in mouse is 5290 mg/kg, and in rat is over 5000 mg/kg. Symptoms of a cycloserine overdose include drowsiness, confusion, headache, dizziness, irritability, numbness and tingling, difficulty speaking, paralysis, abnormal behavior, seizures, and unconsciousness.
Description
D-Cycloserine (68-41-7) is a partial agonist at the glycine modulatory site of NMDA glutamatergic receptors.1?Blocks kainate-induced seizures2?and displays anticonvulsant effects3?in rat models. D-Cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices.4?Second-line drug for the treatment of tuberculosis. ?Enhances activity-dependent plasticity in human adults.5
Description
d-Cycloserine is a naturally occurring cyclic amino acid that can be thought of as an oxidatively cyclized version of its namesake d-serine. It is produced by?Streptomyces?bacteria and has been used to treat the tuberculosis pathogen,?Mycobacterium tuberculosis.
d-Cycloserine, also called oxymycin and?D-4-amino-3-isoxazolidone, was the subject of a flurry of articles published in issue 8 of the 1955 volume of?JACS. In succession, Merck researchers reported its?isolation from?Streptomyces garyphalus; scientists at Eli Lilly and Commercial Solvents Corp. reported?similar results from?Streptomyces ocrhidaceus?; and Merck chemists?synthesized racemic cycloserine.
Fast-forward to 2015: Psychologist Robert S. Asarnow and co-workers at UCLA were building on earlier research in lab animals that showed that?N-methyl-D-aspartate receptor (NMDAR) signaling in the brain is involved in strengthening synapses during learning.
They hypothesized that?D-cycloserine, an NMDAR signal booster, might have the same effects in humans. Using electroencephalography (EEG), they found that healthy subjects treated with the compound?exhibited larger “learning spikes”? in their EEG traces than did untreated individuals.
Psychologist Stefan G. Hoffmann at Boston University says that this finding is an indication that?D-cycloserine or other NMDAR signal enhancers might be useful for enhancing forms of cognition that are relevant to psychiatric disorders.
The Uses of D-Cycloserine
D-Cycloserine inhibits cell wall biosynthesis (D-Ala peptide bond formation). D-Cycloserine also prevents conversion of D-Ala to L-Ala. D-Cycloserine is an bacteriostatic. D-Cycloserine is an antibiot ic against Gram-negative bacteria.
Indications
Used in combination with up to 5 other drugs as a treatment for Mycobacterium avium complex (MAC) and is also used to treat tuberculosis (TB).
Background
Antibiotic substance produced by Streptomyces garyphalus.
What are the applications of Application
D-Cycloserine is antibiotic and a partial agonist of NMDA receptors at the glycine modulatory site
Pharmacokinetics
Cycloserine, a broad-spectrum antibiotic, may be bactericidal or bacteriostatic, depending on its concentration at the site of infection and the susceptibility of the organism. Cycloserine works by blocking the formation of these peptidoglycans. By doing this the walls of the bacteria become weak and it results in the death of the bacteria
Metabolism
Not Available
Properties of D-Cycloserine
| Melting point: | 147 °C (dec.)(lit.) |
| Boiling point: | 191.38°C (rough estimate) |
| Density | 1.3516 (rough estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | water: soluble50mg/mL, clear, colorless to light yellow |
| form | powder |
| color | white to off-white |
| Water Solubility | SOLUBLE |
| Sensitive | Air Sensitive |
Safety information for D-Cycloserine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for D-Cycloserine
Abamectin manufacturer
PRODIGIOUS LIFE SCIENCES
Solara Active Pharma Sciences Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran


You may like
-
 68-41-7 (D-cycloserine) 98%View Details
68-41-7 (D-cycloserine) 98%View Details
68-41-7 -
 68-41-7 99%View Details
68-41-7 99%View Details
68-41-7 -
 Cycloserine 98%View Details
Cycloserine 98%View Details
68-41-7 -
 17673-56-2 99%View Details
17673-56-2 99%View Details
17673-56-2 -
 13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 -
 143-07-7 99%View Details
143-07-7 99%View Details
143-07-7 -
 20776-67-4 99%View Details
20776-67-4 99%View Details
20776-67-4 -
 acid blue 113, acid navy blue , wool navy blue 0View Details
acid blue 113, acid navy blue , wool navy blue 0View Details
3351-05-1