Crizotinib
Synonym(s):(R)-3-[1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine;PF 2341066;PF02341066;PF-02341066;Xalkori
- CAS NO.:877399-52-5
- Empirical Formula: C21H22Cl2FN5O
- Molecular Weight: 450.34
- MDL number: MFCD12407409
- EINECS: 638-814-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-16 18:29:06
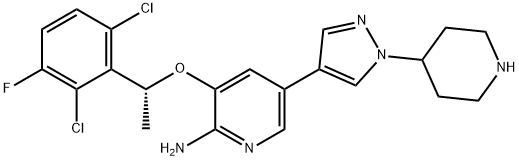
What is Crizotinib?
Absorption
In patients with pancreatic, colorectal, sarcoma, anaplastic large-cell lymphoma and non-small cell lung cancer (NSCLC) treated with crizotinib doses ranging from 100 mg once a day to 300 mg twice a day, the mean AUC and Cmax increased in a dose-proportional manner. A single crizotinib dose of crizotinib is absorbed with a median tmax 4 to 6 hours. In patients receiving multiple doses of crizotinib 250 mg twice daily (n=167), the mean AUC was is 2321.00 ng?hr/mL, the mean Cmax was 99.60 ng/mL, and the median tmax was 5.0 hours. The mean absolute bioavailability of crizotinib is 43%, ranging from 32% to 66%. High-fat meals reduce the AUC0-INF and Cmax of crizotinib by approximately 14%. Age, sex at birth, and ethnicity (Asian vs non-Asian patients) did not have a clinically significant effect on crizotinib pharmacokinetics. In patients less than 18 years old, higher body weight was associated with a lower crizotinib exposure.
Toxicity
The maximum tolerated dose of crizotinib is the same as the recommended dosing regimen (250 mg twice daily). This was defined based on a phase 1 dose-escalation study in patients with advanced solid tumors. The treatment of crizotinib overdoses should consist of symptomatic treatment and other supportive measures. There is no antidote for crizotinib. In vitro and in vivo studies have shown that crizotinib is genotoxic, and the Ames test showed that crizotinib was not mutagenic. Carcinogenicity studies with crizotinib have not been performed.
In female rats, 500 mg/kg/day (approximately 10 times the recommended human dose based on body surface area) of crizotinib for 3 days induced single-cell necrosis of ovarian follicles. In male rats, 50 mg/kg/day of crizotinib (greater than 1.7 times the recommended human dose) for 28 days induced testicular pachytene spermatocyte degeneration.
Description
Crizotinib is a selective tyrosine kinase receptor inhibitor used in the therapy of selected cases of advanced non-small cell lung cancer. It is a dual ATP competitive inhibitor of tyrosine kinases c-MET (Mesenchymal-Epithelial Transition Factor) kinase (cellular IC50=8 nM) and ALK (cellular IC50=20 nM), both of which are important targets for cancer chemotherapy. When crizotinib was tested for selectivity versus other kinases it was found to have enzyme IC50's within 100-fold multiples of c-MET for 13 of the 120 kinases tested. In cellular assays, crizotinib was found to inhibit RON (recepteur d’origine nantais) kinase with a 10-fold selectivity window over c-MET. Altogether, this agent inhibits tumor cell growth.
XALKORI®(crizotinib) is a prescription medicine used to treat people with non-small cell lung cancer (NSCLC) that has spread to other parts of the body and is caused by a defect in either a gene called ALK (anaplastic lymphoma kinase) or a gene called ROS1. It is not known if XALKORI is safe and effective in children.
Description
Crizotinib is a drug being developed by Pfizer to treat lung cancer, particularly non–small cell lung carcinoma that most frequently affects younger nonsmokers or former light smokers. Its mode of action is to inhibit the enzyme anaplastic lymphoma kinase (ALK).?In a recent Phase I/II trial, crizotinib performed exceptionally well, and a Phase III trial is under way.
The Uses of Crizotinib
Crizotinib is a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). Crizotinib is a potential antitumor agent. In August 2011, the United States FDA approved crizotinib for the treatment of anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer (NSCLC).
Background
Crizotinib is a tyrosine kinase receptor inhibitor used for the treatment of anaplastic lymphoma kinase (ALK) or ROS1-positive non-small cell lung cancer (NSCLC) tumors, as well as ALK-positive anaplastic large cell lymphoma (ALCL) and inflammatory myofibroblastic tumor (IMT). By targeting the echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion protein, crizotinib offers robust effectiveness in treating NSCLC in patients with this type of rearrangement. Crizotinib was the first-in-class drug used to treat ALK-positive tumors. Second- and third-generation ALK-tyrosine kinase-inhibitors have overcome many of the pharmacodynamic and genetic resistance mechanisms crizotinib is prone to. Crizotinib was approved by the FDA in 2011, and its use is accompanied by FDA-approved tests used to detect ALK and ROS1 rearrangements.
Indications
Crizotinib is a kinase inhibitor indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are anaplastic lymphoma kinase (ALK) or ROS1-positive as detected by an FDA-approved test. Crizotinib is also indicated for the treatment of relapsed or refractory, systemic anaplastic large cell lymphoma (ALCL) that is ALK-positive in pediatric patients 1 year of age and older and young adults. The safety and efficacy of crizotinib have not been established in older adults with relapsed or refractory, systemic ALK-positive ALCL. Additionally, crizotinib is indicated for the treatment of adult and pediatric patients 1 year of age and older with unresectable, recurrent, or refractory inflammatory myofibroblastic tumor (IMT) that is ALK-positive.
Pharmacokinetics
In a phase I study, 37 patients with a variety of solid-tumor cancers refractory to therapy received 50 to 300 mg of crizotinib daily or twice daily. In this group, two patients with non-small cell lung cancer (NSCLC) exhibiting echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) mutations responded to therapy; therefore, following studies focused on patients with advanced ALK-positive disease. In this group of patients, the 6-month progression-free survival among crizotinib users was approximately 72%. When compared to ALK mutation-positive patients that did not receive crizotinib, ALK mutation-positive patients treated with crizotinib had a higher two-year overall survival rate (54% vs 36%).
The use of crizotinib may lead to hepatotoxicity, interstitial lung disease (ILD), pneumonitis, QT interval prolongation, bradycardia, severe visual loss, ??embryo-fetal toxicity and gastrointestinal toxicity in pediatric and young adult patients with anaplastic large cell lymphoma (ALCL) or pediatric patients with inflammatory myofibroblastic tumor (IMT).
Metabolism
Crizotinib is mainly metabolized in the liver by CYP3A4 and CYP3A5, and undergoes an O-dealkylation, with subsequent phase 2 conjugation. Non-metabolic elimination, such as biliary excretion, can not be excluded. PF-06260182 (with two constituent diastereomers, PF-06270079 and PF-06270080) is the only active metabolite of crizotinib that has been identified. In vitro studies suggest that, compared to crizotinib, PF-06270079 and PF-06270080 are approximately 3- to 8-fold less potent against anaplastic lymphoma kinase (ALK) and 2.5- to 4-fold less potent against Hepatocyte Growth Factor Receptor (HGFR, c-Met).
Properties of Crizotinib
| Melting point: | 192 °C |
| Boiling point: | 599.2±50.0 °C(Predicted) |
| Density | 1.47±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | Soluble in DMSO (up to 25 mg/ml with warming) or in Ethanol (up to 25 mg/ml with warming) |
| form | powder |
| color | white to tan |
Safety information for Crizotinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Crizotinib
| InChIKey | KTEIFNKAUNYNJU-GFCCVEGCSA-N |
| SMILES | C1(N)=NC=C(C2=CN(C3CCNCC3)N=C2)C=C1O[C@@H](C1=C(Cl)C=CC(F)=C1Cl)C |
Abamectin manufacturer
Jigs Chemical ltd
AVD pharmaceuticals Pvt Ltd
Bulat Pharmaceutical Pvt Ltd
Apothecon Pharmaceuticals Pvt Ltd
Shreyaa Medilife Private Limited
Aspen Biopharma Labs Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol 3-NITRO-5-ACETYL IMINODIBENZYL (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran



You may like
-
 877399-52-5 Crizotinib 98%View Details
877399-52-5 Crizotinib 98%View Details
877399-52-5 -
 877399-52-5 98%View Details
877399-52-5 98%View Details
877399-52-5 -
 Crizotinib 98%View Details
Crizotinib 98%View Details
877399-52-5 -
 Crizotinib 877399-52-5 99%View Details
Crizotinib 877399-52-5 99%View Details
877399-52-5 -
 877399-52-5 Crizotinib 98%View Details
877399-52-5 Crizotinib 98%View Details
877399-52-5 -
 877399-52-5 98%View Details
877399-52-5 98%View Details
877399-52-5 -
 877399-52-5 98%View Details
877399-52-5 98%View Details
877399-52-5 -
 Crizotinib 99%View Details
Crizotinib 99%View Details