Chloroform
Synonym(s):Methylidyne trichloride;TCM, Trichloromethane, Methane trichloride, Methyl trichloride;Trichloromethane;Trichloromethane, Chloroform;Trichloromethane, FM approval
- CAS NO.:67-66-3
- Empirical Formula: CHCl3
- Molecular Weight: 119.38
- MDL number: MFCD00000826
- EINECS: 200-663-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-14 15:18:26
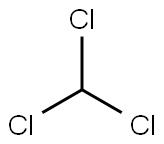
What is Chloroform?
Description
Chloroform is a trichlorinated organic substance with the chemical formula of CHCl3. It is a clear liquid at room temperature and has a pleasant, sweet odor. Chloroform is only slightly soluble in water and evaporates quickly into surrounding air, increasing the risk of inhalation exposure. In addition, chloroform persists for a long time in both water and air. There are no natural sources of chloroform, but this contaminant enters the environment through a variety of industrial operations, including the chlorination of water. Humans can be exposed to chloroform through inhalation, ingestion, and dermal contact.
Chloroform has a relatively narrow margin of safety and has been replaced by better inhalation anesthetics. In addition, it is believed to be toxic to the liver and kidneys and may cause liver cancer. Chloroform was once widely used as a solvent, but safety and environmental concerns have reduced this use as well. Nevertheless, chloroform has remained an important industrial chemical.
Description
Chloroform (CHCl3) is a relatively common organic solvent. In most organic chemistry labs, however, methylene chloride is used as a substitute. Deuterated chloroform (CDCl3) is one of the most common NMR solvents.
The Uses of Chloroform
Chloroform is a widely used industrial and laboratory solvent. It is a volatile chlorinated organic solvent whose vapors have a narcotic effect.Due to its light sensitivity, it may undergo degradation with time. This can be suppressed by adding ethanol as a stabilizer. The addition will increase the polarity of the solvent and potentially impact certain applications.
- Chloroform has been used as a solvent for dissolving lipids and also as a cleansing agent.
- It may also be used in solvent extraction process and also for recrystallization.
- Suitable for HPLC, spectrophotometry, environmental testing
- Facilitates recovery of the aqueous phase of PCRs which have been overlaid with mineral oil.
- Meets ACS specifications.
The Uses of Chloroform

To a solution of the SM (80 g, 0.27 mol) in CHCl3 (1 L) at 10-15 C was added dropwise a solution of Br2 (16 mL, 0.31 mol) in CHCl3 (100 mL). The reaction was stirred at 12 C for 10 min, then washed sequentially with cooled sat aq NaHCO3 (2 x 500 mL) and sat aq brine (400 mL), dried, and concentrated in vacuo. Crystallization of the residue from EtOAc provided the product as a yellow solid. [90 g, 88%]
What are the applications of Application
Chloroform is a relatively unreactive common laboratory solvent
Background
Chloroform is an organic small molecule, member of the family of the chloromethanes that presents a formula of CHCl3. It is a colorless, sweet-smelling, dense liquid that is produced on a large scale as a precursor to PTFE and refrigerants, but the latter application is declining. Chloroform is part of the FDA drug products withdrawn or removed from the market for reasons of safety or effectiveness.
Metabolism
Not Available
Properties of Chloroform
| Melting point: | -63 °C |
| Boiling point: | 61 °C |
| Density | 1.492 g/mL at 25 °C(lit.) |
| Flash point: | 60.5-61.5°C |
| storage temp. | 2-8°C |
| solubility | Miscible with diethyl ether, oils, ligroin, alcohol, carbon tetrachloride and carbon disulfide. |
| form | Liquid |
| appearance | Colorless liquid |
| color | ≤10(APHA) |
| Odor | Ethereal, sweet odor detectable at 133 to 276 ppm (mean = 192 ppm) |
| Water Solubility | 8 g/L (20 ºC) |
Safety information for Chloroform
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Chloroform
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran





You may like
-
 2,3 Dichloro-4-Hydroxy Aniline 39183-17-0 99%View Details
2,3 Dichloro-4-Hydroxy Aniline 39183-17-0 99%View Details
39183-17-0 -
 17673-56-2 99%View Details
17673-56-2 99%View Details
17673-56-2 -
 13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 -
 143-07-7 99%View Details
143-07-7 99%View Details
143-07-7 -
 Ethanolamine 141-43-5 99%View Details
Ethanolamine 141-43-5 99%View Details
141-43-5 -
 616-47-7 99%View Details
616-47-7 99%View Details
616-47-7 -
 20776-67-4 99%View Details
20776-67-4 99%View Details
20776-67-4 -
 acid blue 113, acid navy blue , wool navy blue 0View Details
acid blue 113, acid navy blue , wool navy blue 0View Details
3351-05-1