CALCIUM
Synonym(s):Atomic calcium;CA006101;Calcium atom;Calcium element
- CAS NO.:14452-75-6
- Empirical Formula: Ca
- Molecular Weight: 40.08
- MDL number: MFCD00085314
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-11-28 16:31:43
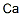
What is CALCIUM?
Description
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic weight of 40.078 g/mol. Calcium is the fifth most abundant dissolved ion in seawater by both molarity and mass, after Na+, Cl-, Mg2+ and SO42-. Calcium metal is quite reactive. It readily forms a white coating of calcium nitride (Ca3N2) in air at room temperature. It reacts with water and the metal burns in air with an orangered flame, forming largely the nitride, but some oxide. In the visible portion of the spectrum of many stars, including the Sun, strong absorption lines of singly ionized calcium ions are evident. Prominent among these are the H line at 3968.5 A ° and the K line at 3933.7 A°of singly ionized calcium, or Ca II. For the Sun, and stars with low temperatures, the prominence of the H and K lines is an indication of strong magnetic activity in the chromosphere. Measurement of periodic variations of these active regions can also be used to deduce the rotational periods of these stars.
The Uses of CALCIUM
Calcium is an alkaline earth element that contributes toward bone and teeth formation, muscle contraction, and blood clotting. it occurs in milk, vegetables, and egg yolk.
Properties of CALCIUM
| form | Liquid |
| color | Clear colorless |
| Water Solubility | Miscible with water. |
Safety information for CALCIUM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases |
| Precautionary Statement Codes |
P231+P232:Handle under inert gas. Protect from moisture. |
Computed Descriptors for CALCIUM
Abamectin manufacturer
Jeevan Chemicals and Pharmaceuticals
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine N-Benzyl-3-hydroxypiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran
You may like
-
 Calcium 99%View Details
Calcium 99%View Details -
 2-Bromo-5-Iodopyridine 73290-22-9 98%View Details
2-Bromo-5-Iodopyridine 73290-22-9 98%View Details
73290-22-9 -
 141-86-6 98%View Details
141-86-6 98%View Details
141-86-6 -
 4-Amino-2-Chloropyridine 14432-12-3 98%View Details
4-Amino-2-Chloropyridine 14432-12-3 98%View Details
14432-12-3 -
 16867-03-1 2-Amino-3-Hydroxypyridine 98%View Details
16867-03-1 2-Amino-3-Hydroxypyridine 98%View Details
16867-03-1 -
 2,3-Diamino-5-Chloropyridine 25710-20-7 98%View Details
2,3-Diamino-5-Chloropyridine 25710-20-7 98%View Details
25710-20-7 -
 13466-41-6 98%View Details
13466-41-6 98%View Details
13466-41-6 -
 Ammonium HexachloropalladateView Details
Ammonium HexachloropalladateView Details
19168-23-1