Barium hydroxide
Synonym(s):Barium dihydroxide
- CAS NO.:17194-00-2
- Empirical Formula: BaH2O2
- Molecular Weight: 171.34
- MDL number: MFCD00003443
- EINECS: 241-234-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-21 15:24:59
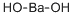
What is Barium hydroxide ?
Description
Barium Hydroxide occurs as an anhydrate whose prismatic, colorless
crystals are very deliquescent. Barium hydroxide can
be prepared by dissolving barium oxide (BaO) in water:
BaO+ 9H2O→Ba(OH)2·8H2O
It crystallizes as the octahydrate, which converts to
the monohydrate upon heating in air. Barium oxide
has been found to react with water vapor at temperatures
from 1443 to 1593 K to form gaseous Ba(OH)2
according to the reaction:
BaO(solid)+H2O(gas)→Ba(OH)2(gas)
A better method of preparation involves addition of
NaOH to the nitrate. Since barium hydroxide is fairly
soluble, it is necessary to evaporate the solution to crystallize Ba(OH)2·8H2O. Ethanol is added to aid in this crystallization. The crystals are washed in cold ethanol to remove sodium ions and then dried.
Description
The octahydrate of barium hydroxide is freely soluble in water and MeOH, whereas the monohydrate is only slightly soluble in water. For this reason the octahydrate is the most common form.
The Uses of Barium hydroxide
Barium hydroxide is used in analytical chemistry to titrate weak acids, particularly organic acids. It forms clear aqueous solutions that are free from carbonate, unlike those of the alkali hydroxides, since barium carbonate is insoluble in water. This allows the use of indicators such as phenolphthalein or thymophthalein (with alkaline color changes) without the risk of titration errors due to the presence of weakly basic CO32- ions.Barium hydroxide is used in organic synthesis as a strong base, for example for the hydrolysis of esters and nitriles.It has been used to hydrolyze one of the two equivalent ester groups in dimethyl endecanedioate. It is also used in the preparation of cyclopentanone, diacetone alcohol and d-Gluonic g-lactone.
Barium hydroxide is used as an additive in thermoplastics (such as phenolic resins), rayon and PVC stabilizers to improve plastic properties. This material is used as a general-purpose additive for lubricants and greases. Other industrial applications for barium hydroxide include sugar fabrication, manufacturing soaps, fat saponification, fusing of silicates and chemical synthesis of other barium compounds and organic compounds.
Barium hydroxide is offered for sale both as the octahydrate and the monohydrate. It is available in large quantities commercially.
The Uses of Barium hydroxide

To a solution of KOH (10 g, 178 mmol) in H2O (15 mL) and EtOH (15 mL) at RT was added the SM (3.92 g, 42 mmol) over 10 min. The reaction mixture was stirred at 90 C for 3.5 h, after which time it was concentrated in vacuo. The resulting residue was dissolved in H2O (10 mL) and acidified at 0 C with 6M HCl to pH 1. The mixture was extracted with DCM. The org layer was dried (MgSO4) and concentrated to provide the product as a colorless oil which was taken forward without further purification. [4.65 g, 98%]
Properties of Barium hydroxide
| Melting point: | >300 °C(lit.) |
| Boiling point: | decomposes at 1032 (calculated) [KNA91] |
| Density | 2.2 g/mL at 25 °C(lit.) |
| form | Solid |
| appearance | White solid (monohydrate) |
| color | Clear |
| Odor | no odor |
| Water Solubility | Soluble in water. |
| Sensitive | Air Sensitive & Hygroscopic |
Safety information for Barium hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Barium hydroxide
| InChIKey | RQPZNWPYLFFXCP-UHFFFAOYSA-L |
Abamectin manufacturer
ALLIPO CHEMICALS
Kronox Lab Sciences Pvt Ltd
Nithyasri Chemicals (APURVA CHEMICALS)
Divjyot Chemicals Private Limited
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine N-Benzyl-3-hydroxypiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran



You may like
-
 17194-00-2 Barium Hydroxide 97%View Details
17194-00-2 Barium Hydroxide 97%View Details
17194-00-2 -
 Barium hydroxide 98%View Details
Barium hydroxide 98%View Details -
 Barium hydroxide 98%View Details
Barium hydroxide 98%View Details
17194-00-2 -
 Barium hydroxide 17194-00-2 98%View Details
Barium hydroxide 17194-00-2 98%View Details
17194-00-2 -
 17194-00-2 99%View Details
17194-00-2 99%View Details
17194-00-2 -
 4-Amino-2-Chloropyridine 14432-12-3 98%View Details
4-Amino-2-Chloropyridine 14432-12-3 98%View Details
14432-12-3 -
 13466-41-6 98%View Details
13466-41-6 98%View Details
13466-41-6 -
 Ammonium HexachloropalladateView Details
Ammonium HexachloropalladateView Details
19168-23-1