Anastrozole
Synonym(s):Anastrozole;Arimidex;2;2"-[5-(1H-1;2;4-Triazol-1-ylmethyl)-1;3-Phenylene]bis(2-methyl-propiononitrile;a,a,a′,a′-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile
- CAS NO.:120511-73-1
- Empirical Formula: C17H19N5
- Molecular Weight: 293.37
- MDL number: MFCD00866298
- EINECS: 601-715-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-30 16:33:02
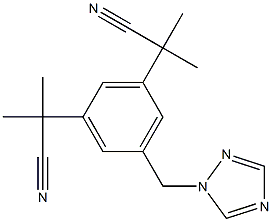
What is Anastrozole?
Absorption
Anastrozole is rapidly absorbed and Tmax is typically reached within 2 hours of dosing under fasted conditions. Coadministration with food reduces the rate but not the overall extent of absorption - mean Cmax decreased by 16% and the median Tmax was extended to 5 hours when anastrozole was administered 30 minutes after ingestion of food, though this relatively minor alteration in absorption kinetics is not expected to result in clinically significant effects.
Toxicity
The reported oral TDLo in a human woman is 1.68 mg/kg given intermittently over the course of 12 weeks. Knowledge of the signs and symptoms of anastrozole overdose is incomplete as there are no documented descriptions of a patient receiving more than 60mg, a dose which was administered to a healthy male volunteer and was well-tolerated. There is no antidote for anastrozole and treatment should be supportive and symptomatic, including close monitoring of patient vital signs. As anastrozole exhibits relatively low protein binding, dialysis may be helpful and should be considered in select cases.
Description
Anastrozole entered its first market in the United Kingdom for the treatment of advanced breast cancer in post-menopausal women. Anastrozole is a highly potent and selective aromatase inhibitor. It is extremely potent in lowering circulating estradiol to undetectable levels in treated patients without altering other circulating hormones. The drug is reportedly well absorbed and tolerated following oral administration.
The Uses of Anastrozole
Anastrozole is a nonsteroidal inhibitor of aromatase which effectively blocks estrogen synthesis in postmenopausal women and is used as therapy of estrogen receptor positive breast cancer. Anastrozole has been associated with a low rate of serum enzyme elevations during therapy and rare instances of clinically apparent liver injury.
Background
Anastrozole is a non-steroidal aromatase inhibitor (AI), similar to letrozole, used to decrease circulating estrogen levels in the treatment of postmenopausal women with estrogen-responsive breast cancer. Aromatase inhibitors, including anastrozole, have become endocrine drugs of choice in the treatment of postmenopausal breast cancer due to a more favourable efficacy and adverse effect profile as compared to earlier estrogen receptor modulators such as tamoxifen.
Indications
Anastrozole is indicated as adjunct therapy in the treatment of hormone receptor-positive early breast cancer in postmenopausal women, and as a first-line treatment for hormone receptor-positive (or hormone receptor-unknown) locally advanced or metastatic breast cancer in postmenopausal women. It may also be used in the treatment of advanced breast cancer in postmenopausal women who experience disease progression despite treatment with tamoxifen.
What are the applications of Application
Anastrozole is a CYP19 (aromatase) inhibitor
Pharmacokinetics
Anastrozole prevents the conversion of adrenal androgens (e.g. testosterone) to estrogen in peripheral and tumour tissues. As the growth of many breast cancers is stimulated and/or maintained by the presence of estrogen, anastrozole helps to treat these cancers by decreasing the levels of circulating estrogens.
The incidence of ischemic cardiovascular events was increased during anastrozole therapy and patients with pre-existing ischemic heart disease should consider the risks and benefits of anastrozole before beginning therapy. Anastrozole has also been reported to decrease spine and hip bone mineral density (BMD), so consideration should be given to monitoring of BMD in patients receiving long-term therapy.
Metabolism
Anastrozole is primarily metabolized in the liver via oxidation and glucuronidation to a number of inactive metabolites, including hydroxyanastrozole (both free and glucuronidated) and anastrozole glucuronide. Oxidation to hydroxyanastrozole is catalyzed predominantly by CYP3A4 (as well as CYP3A5 and CYP2C8, to a lesser extent) and the direct glucuronidation of anastrozole appears to be catalyzed mainly by UGT1A4.
Anastrozole may also undergo N-dealkylation to form triazole and 3,5-Bis-(2-methylpropiononitrile)-benzoic acid. Labels for anastrozole state the main metabolite found in plasma following administration is triazole, but a recent pharmacokinetic study was unable to detect any products of N-dealkylation in vitro.
Side Effects
The side effects of Anastrozole including hot flashes, headache, bone pain, trouble sleeping, dizziness, stomach upset, nausea/vomiting, constipation, diarrhea, loss of appetite, weight gain, tiredness/weakness, increased coughing, or sore throat may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.
Properties of Anastrozole
| Melting point: | 81-82°C |
| Boiling point: | 469.7±55.0 °C(Predicted) |
| Density | 1.08±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: soluble40mg/mL |
| form | solid |
Safety information for Anastrozole
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Anastrozole
| InChIKey | YBBLVLTVTVSKRW-UHFFFAOYSA-N |
Abamectin manufacturer
Jigs Chemical ltd
GLP Pharma Standards
AVD pharmaceuticals Pvt Ltd
SGMR PHARMACEUTICALS PVT LTD
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran

You may like
-
 AnastrazoleAPI & intermediates 98%View Details
AnastrazoleAPI & intermediates 98%View Details
120511-73-1 -
 Anastrozole 98%View Details
Anastrozole 98%View Details -
 Anastrozole 98%View Details
Anastrozole 98%View Details
120511-73-1 -
 120511-73-1 98%View Details
120511-73-1 98%View Details
120511-73-1 -
 120511-73-1 99%View Details
120511-73-1 99%View Details
120511-73-1 -
 Anastrozole 120511-73-1 98%View Details
Anastrozole 120511-73-1 98%View Details
120511-73-1 -
 120511-73-1 Anastrozole 98%View Details
120511-73-1 Anastrozole 98%View Details
120511-73-1 -
 120511-73-1 Anastrozole 99%View Details
120511-73-1 Anastrozole 99%View Details
120511-73-1