

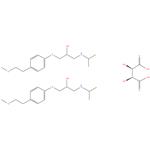
Metoprolol tartrate 98%
| Price | Get Latest Price | ||||
| Packge | 1MT | 1kg | 5kg | 10kg | 25kg |
- Min. Order:1MT
- Time:2024-01-22
Product Details
- Product NameMetoprolol tartrate
- CAS No.56392-17-7
- EINECS No.260-148-9
- MF2C15H25NO3.C4H6O6
- MW684.82
- Appearancepowderwhite to off-white
- storage temp. 2-8°C
- Water Solubility Soluble in water (>1000 mg/ml), methanol (>500 mg/ml), chloroform (496 mg/ml), ethanol (31 mg/ml at 25°C), and DMSO (100 mg/ml at 25°C).
- Melting point 120℃
- Boiling point 695.37°C (rough estimate)
- density 1.1946 (rough estimate)
Company Profile Introduction
Vani Pharma Labs Limited, Hyderabad along with its sister concern Vani Organics Private limited, Bidar is one of the pioneering companies in the Indian bulk drug industry. Incorporated in June 1976 by a team of visionary technocrats of the period, Vani began and grew from strength to strength in Active Pharmaceutical Ingredients (APIs) and bulk drugs manufacturing. Commencing with the manufacture of Analgin, a basic analgesic drug and its intermediates, the group has substantially expanded and diversified into a diverse portfolio of Agro, Industrial, Pharmaceutical Intermediates, fine and specialty chemicals.
Its operational plants are located in a sprawling 2-acre land in Phase I of the Jeedimetla Industrial Development Area, Hyderabad, India and an 8-acre land at Bidar, India. Vani is known for its quality products, exported to over seven countries across the world, in compliance to various international standards. The Company regularly partners with Research & Development projects of reputed Pharma Companies for development and stabilization of molecules.
The Company has an installed capacity of 160 KL in Hyderabad and 100 KL in Bidar to manufacture Bulk drugs and API Intermediates. The Company has dedicated Quality Control, Quality Assurance and R&D departments. The Quality system is in compliance with ICH q7 guidelines. The company holds WHO GMP (CDSCO), ISO 9001:2015 & ISO 14001:2015 Certifications for its APIs. The Company holds required approvals from Pollution Control Board to manufacture APIs and intermediates in the quantities of 160 MT/month in Hyderabad and 800 MT/month in Bidar. Vani currently disposes effluents to the Common Effluent Treatment Plant (JETL) in which, the Company is a founder member.
Supplier other products
Recommended supplier
-
VIP1年
- SETV ASRV LLP
- METOPROLOL TARTRATE 95-99 %
- Inquiry
- 2024-09-11
-
VIP0年
- Ebenezer Industries
- 56392-17-7 98%
- Inquiry
- 2024-03-30
-
VIP1年
- Exemed Pharmaceuticals (Oneiro Chemicals Pvt Ltd)
- Metoprolol tartrate 98%
- Inquiry
- 2024-02-28
-
VIP1年
- Globela Industry Pvt Ltd
- 56392-17-7 98%
- Inquiry
- 2024-02-22
-
VIP1年
- Reine Lifescience
- 56392-17-7 Metoprolol tartrate 98%
- Inquiry
- 2024-02-09
-
VIP1年
- Ralington Pharma
- Metoprolol tartrate 56392-17-7 98%
- Inquiry
- 2024-02-05
-
VIP1年
- Afton Pharma
- 56392-17-7 98%
- Inquiry
- 2024-01-19
-
VIP1年
- Kopran Ltd
- 56392-17-7 Metoprolol tartrate 98%
- Inquiry
- 2024-01-19
-
VIP1年
- CTX Lifesciences Pvt Ltd
- Metoprolol tartrate 56392-17-7 99%
- Inquiry
- 2024-01-10
-
VIP1年
- Sun Pharmaceutical Industries Ltd
- Metoprolol tartrate 98%
- Inquiry
- 2024-01-09
- Since:2024-01-01
- Address: "Plot No: 11 & 12, Phase-1,_x000D_ Industrial Development Area,_x000D_ Jeedimetla, Hyderabad, Tela



