

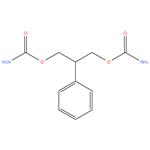
Felbamate 98%
| Price | Get Latest Price | ||||
| Packge | 1MT | 1kg | 5kg | 10kg | 25kg |
- Min. Order:1MT
- Time:2024-03-07
Product Details
- Product NameFelbamate
- CAS No.25451-15-4
- EINECS No.247-001-4
- MFC11H14N2O4
- MW238.24
- AppearanceSolidWhite
- Melting point 148-1500C
- storage temp. Keep in dark place,Inert atmosphere,Room temperature
- Boiling point 511.9±50.0 °C(Predicted)
- density 1.275±0.06 g/cm3(Predicted)
Company Profile Introduction
Apicore US LLC headquartered in New Jersey, USA, and its contract manufacturing organization, Apicore Pharmaceuticals Pvt Ltd (Gujarat, India) are dedicated to developing and manufacturing active pharmaceutical ingredients (APIs) at our facilities, located in Somerset, NJ and Gujarat, India for the worldwide pharmaceutical industry.
Apicore LLC, a wholly owned subsidiary of RK Pharma Inc (www.rkpharmainc.com), is a leading process R&D and API manufacturing service provider for the worldwide pharmaceutical industry. We offer a wide portfolio of services ranging from API’s for the generic industry to custom synthesis for early phase pharmaceutical research and branded products.
Our USFDA approved facilities in India (Visakhapatnam, Andhra Pradesh and Vadodara, Gujarat) are both equipped with state-of-the-art analytical and research capabilities.
While our worldwide network of locations seamlessly integrate with each other, each unit also independently houses a full suite of R&D, QA, QC, and Manufacturing Services. This facilitates a continuous real-time exchange of information, ideas, and data while eliminating redundancies and waste.
Supplier other products
Recommended supplier
-
VIP1年
- Mylan Laboratories Ltd
- 25451-15-4 98%
- Inquiry
- 2024-03-06
-
VIP1年
- Solara Active Pharma Sciences Ltd
- 25451-15-4 Felbamate 98%
- Inquiry
- 2024-01-23
-
VIP1年
- Arch Pharmalabs Ltd
- Felbamate 25451-15-4 98%
- Inquiry
- 2024-01-22
-
VIP1年
- Hetero Drugs Limited
- Felbamate 98%
- Inquiry
- 2024-01-08
- Since:2021-03-01
- Address: Block no. 252-253, Opp. Jain Irrigation Co., Padra-Jambusar Highway, Tal. Padra,Village Dhobikuva, V



