

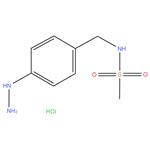
88933-16-8 N-Methyl-4-diazanylsulfabenzamide 98%
| Price | Get Latest Price | ||||
| Packge | 1MT | 1kg | 5kg | 10kg | 25kg |
- Min. Order:1MT
- Time:2023-09-15
Product Details
- Product NameN-Methyl-4-diazanylsulfabenzamide
- CAS No.88933-16-8
- MFC8H14ClN3O2S
- MW251.73
- AppearanceSolidPale Yellow to Pale Beige
- Melting point 170-173°C
- storage temp. Inert atmosphere,2-8°C
Company Profile Introduction
THE STORY SO FAR
The year 1994
Porus was started with one manufacturing location in the year 1994 to manufacture N-Acetyl Sulphanilyl Chloride (N-ASC), which is a key intermediate for all Sulpha based drugs.
The year 1998
With strong technology back up and aggressive marketing the company became the largest source of N-ASC in India displacing the then market leader tama, a Japanese company. The second unit was added in 1998 by taking over a sick unit at Bibinagar about 40 kms from Hyderabad to manufacture sulphamethaxazole, which was a natural integration forward to the intermediate N-ASC. However due to unfavorable market conditions the project was dropped. However the unit continued to support domestic API manufacturers for their intermediates like QAcid-Ciprofloxacin intermediate (40 Mt/month).
The year 1999
The third unit was added in the year 1999 at Kodad on the Vijayawada highway, another sick unit taken over and turned around to meet the growing demand of NASC (300 Mt/month) and other intermediates like Iso Butyl Acetophenone – Ibuprofen intermediate (120 Mt/month) etc.
The year 2000
Around the year 2000 R&D was started at Jeedimetla to develop our own intermediates with efficient processes with emphasis on antimigraine category of drugs called triptans. Since then porus has come to be known as the worldwide leader in this category and enjoys significant market share in the domestic market catering to customers like DR.REDDYS, SUNPHARMA, LUPIN, TEVA etc.
The year 2006
The acquisition of our fourth unit happened in the year 2006 to cater to the speciality chemicals business that had just started to shape up with our efforts in R&D. Using our extensive knowledge both in process as well as scale up we could grow this business. Today this is growing at about 50% yoyo with strong product line.
The year 2013
We have started commencement of work at our Unit-V, Pharmacity, Visakhapatnam with strong emphasis on APIs. We are in the process of building the state of the art API manufacturing facility which will commence production from the mid of 2014.
Recommended supplier
-
VIP1年
- Lakshmidurga Drugs And Intermediates Pvt Ltd
- 88933-16-8 98%
- Inquiry
- 2024-02-26
-
VIP1年
- Maithili Life Sciences Pvt Ltd
- 88933-16-8 4-Hydrazino-N-methylbenzenemethanesulfonamide hydrochloride 98%
- Inquiry
- 2024-02-23
-
VIP1年
- Rui Laboratories Pvt Ltd
- 4-Hydrazino-N-methylbenzenemethanesulfonamide hydrochloride 88933-16-8 99%
- Inquiry
- 2024-02-14
-
VIP1年
- Synergene Active Ingredients Pvt. Ltd.
- 4-Hydrazino-N-methylbenzenemethanesulfonamide hydrochloride 98%
- Inquiry
- 2024-01-17
-
VIP1年
- Lakshmi Farmachem
- 4-Hydrazino-N-methylbenzenemethanesulfonamide hydrochloride 98%
- Inquiry
- 2024-01-11
-
VIP1年
- Viyash Life Sciences Pvt Ltd
- 88933-16-8 4-Hydrazino-N-methylbenzenemethanesulfonamide hydrochloride 98%
- Inquiry
- 2024-01-05
-
VIP1年
- Ability Life Sciences
- 4-Hydrazino-N-methylbenzenemethanesulfonamide hydrochloride 98%
- Inquiry
- 2024-01-05
-
VIP1年
- Varanous Labs Pvt Ltd
- Sumatriptan Hydrazine Impurity 98%
- Inquiry
- 2023-12-26
- Since:2020-09-01
- Address: "Plot No.5,6,15 & 16, Kavuri Hills, Road No.36, Jubliee Hills, Hyderabad - 500 033. Telangana., INDI
