



Cupric oxalate
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2020-01-08
Product Details
- Product NameCupric oxalate
- CAS No.814-91-5
- EINECS No.212-411-4
- MFC2CuO4
- MW151.56
- InChIKeyQYCVHILLJSYYBD-UHFFFAOYSA-L
- AppearancePowderblue
- Water Solubility insoluble
- Melting point anhydrous decomposes at ~300 to copper oxide [HAW93]
- storage temp. Refrigerator, under inert atmosphere
▼
▲
Cupric oxalate Basic information
▼
▲
Product Name:
Synonyms:
Kupfer(II)-oxalat;Copper(Ⅱ)oxalate hemihydrate;Cupric oxalate;Cupric oxalate (1:1);Nsc86015;Copper(II) oxalate hydrate;Copperoxalatehemihydratebluepowder;coppergluconatechelate
CAS:
MF:
C2CuO4
MW:
151.57
EINECS:
212-411-4
Product Categories:
Mol File:
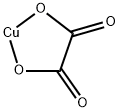
▼
▲
Cupric oxalate Chemical Properties
▼
▲
Melting point
anhydrous decomposes at ~300 to copper oxide [HAW93]
form
Powder
color
blue
Water Solubility
insoluble
Solubility Product Constant (Ksp)
pKsp: 9.35
CAS DataBase Reference
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Risk Statements
Safety Statements
RIDADR
UN 2449
HazardClass
6.1(b)
PackingGroup
III
Hazardous Substances Data
▼
▲
Cupric oxalate Usage And Synthesis
▼
▲
Chemical Properties
Cupric oxalate is a bluish-white, odorless powder.
Uses
As catalyst for organic reactions; as stabilizer for acetylated polyformaldehyde; in anticaries compositions; in seed treatments to repel birds and rodents.
General Description
Odorless bluish-white solid. Denser than water and insoluble in water. Hence sinks in water. Used as a catalysts for organic reactions.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Cupric oxalate dissolves in aqueous ammonia and reacts as an acid to neutralize other bases as well. Can serve as a reducing agent in reactions that generate carbon dioxide.
Hazard
Toxic by ingestion; tissue irritant.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion of very large amounts may produce symptoms of oxalate poisoning; watch for edema of the glottis and delayed constriction of esophagus. Contact with eyes causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic carbon monoxide gas may form in fire.
Potential Exposure
Used as a catalyst for organic reactions and in seed treatment as a repellent for birds and rodents.
Shipping
UN2775, Copper based pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Explosive materials are formed on contact with acetylene gas, ammonia, caustic solutions; sodium hypobromite, nitromethane. Slight heating can cause a weak explosion. Cupric oxalate dissolves in aqueous ammonia and reacts as an acid to neutralize other bases as well. Can serve as a reducing agent in reactions that generate carbon dioxide. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
Recommended supplier
-
VIP1年
- New Alliance Dye Chem Pvt Ltd
- 814-91-5 Cupric oxalate 98%
- Inquiry
- 2024-01-09
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
