

FENOTHIOCARB
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:1ton
- Time:2020-01-01
Product Details
- Product NameFENOTHIOCARB
- CAS No.62850-32-2
- EINECS No.200-589-5
- MFC13H19NO2S
- MW253.36
- Appearanceneat
- storage temp. 0-6°C
- Melting point 40.5℃
- density 1.1414 (rough estimate)
- Water Solubility 30 mg l-1 (20 °C)
- Boiling point 155 °C
▼
▲
FENOTHIOCARB Basic information
▼
▲
Product Name:
Synonyms:
bi-5452;dimethylcarbamothioicacids-(4-phenoxybutyl)ester;dimethyl-carbamothioicacis-(4-phenoxybutyl)ester;kco-3001;phenothiocarb;s-4-phenoxybutyldimethylthiocarbamate;FENOTHIOCARB;PANOCON
CAS:
MF:
C13H19NO2S
MW:
253.36
EINECS:
Product Categories:
Mol File:
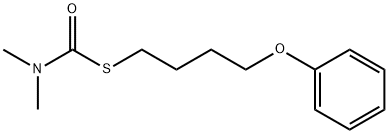
▼
▲
FENOTHIOCARB Chemical Properties
▼
▲
Melting point
40.5℃
Boiling point
155 °C
density
1.1414 (rough estimate)
vapor pressure
1.6 x l0-5 Pa (23 °C)
refractive index
1.6800 (estimate)
storage temp.
0-6°C
pka
-1.21±0.70(Predicted)
form
neat
Water Solubility
30 mg l-1 (20 °C)
BRN
2125738
▼
▲
Safety Information
▼
▲
FENOTHIOCARB Usage And Synthesis
▼
▲
Uses
Fenothiocarb is an acaricide used to control the eggs and young stages of Panonychus citri, Panonychus ulmi and other Panonychus spp.
Metabolic pathway
When the red mite, applied with 14C-fenothiocarb by the contact method, metabolizes fenothiocarb, resulting in several metabolites via the primary oxidation of the N-methyl moiety. The photodegradation of fenothiocarb on silica gel plate exposed to sunlight gives rise to several degradation products. A primary photochemical reaction seems to be the oxidation of the sulfur atom to form its sulfoxide. Under greenhouse conditions, when fenothiocarb is applied to the citrus trees, the major metabolites identified in the leaves, rinds, and edible fruit are 6-O-malonyl-b-D-glucosode of N- hydroxymethyl fenothiocarb, N-formylfenothiocarb, and glucoside conjugate of phenol (not shown in the map), respectively. In soils, fenothiocarb is more rapidly degraded under upland conditions than under flooded conditions. Main degradation pathways include oxidation of the sulfur atom which results in the formation of methyl-4-phenoxybutylsulfoxide, its sulfone, and 4-phenoxybutylsulfonic acid.
Degradation
Fenothiocarb has long residual activity. It is stable to hydrolysis for 5 days (pH 5-9, 40 °C) but it is decomposed slowly by sunlight (PM). [U-14C-phenyl]Fenothiocarb was applied to the origin of silica gel TLC plates and exposed to sunlight in September or October. The plates were developed, with authentic standard compounds also applied, in two dimensions using different solvent systems. After 72 hours exposure to sunlight, 34% of parent fenothiocarb remained (DT50 45 hours). Twelve photoproducts were detected. Products that were identified are illustrated in Scheme 1 and were N-formyl-fenothiocarb (2), desmethyl-fenothiocarb (3) the bis(4-phenoxybutyl) thiolsulfinate (4), the bis(4-phenoxybutyl) thiolsulfonate (5), the sulfoxide derivative (6) and 4-phenoxybutanesulfonic acid (7). The latter two compounds were major products. The primary photochemical reaction was oxidation of sulfur to form fenothiocarb sulfoxide (6) followed by cleavage of the ester linkage and the oxidation or dimerisation of the 4-phenoxybutanesulfenic acid intermediate. The second main route of photodegradation was oxidation of the N-methyl moiety of fenothiocarb (Unai and Tomiwaza, 1986b).
▼
▲
FENOTHIOCARB Preparation Products And Raw materials
▼
▲
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
