

ETHIOFENCARB
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:1ton
- Time:2020-01-01
Product Details
- Product Name ETHIOFENCARB
- CAS No.29973-13-5
- EINECS No.200-589-5
- MFC11H15NO2S
- MW225.31
- InChIKeyHEZNVIYQEUHLNI-UHFFFAOYSA-N
- Appearanceneat
- storage temp. APPROX 4°C
- density 1.231 g/cm3 (20 ºC)
- Melting point 43-45℃
- Water Solubility 1800 mg l-1 (20 °C)
- Boiling point 327.3±34.0 °C(Predicted)
▼
▲
ETHIOFENCARB Basic information
▼
▲
Product Name:
Synonyms:
2-(ethylthiomethyl) pheyyl-N-emthylcarbamate;Ethiofencarb 100mg [29973-13-5];2-[(ETHYLTHIO)METHYL]PHENYL METHYLCARBAMATE;A-ETHYLTHIO-O-TOLYLMETHYL CARBAMATE;CRONETON;CRONETON(R);Ethiophencarp;ETHIOFENCARB
CAS:
MF:
C11H15NO2S
MW:
225.31
EINECS:
249-981-9
Product Categories:
Mol File:
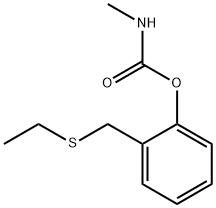
▼
▲
ETHIOFENCARB Chemical Properties
▼
▲
Melting point
43-45℃
Boiling point
327.3±34.0 °C(Predicted)
density
1.231 g/cm3 (20 ºC)
vapor pressure
4.5 x 10-4 Pa (20 °C)
refractive index
1.4790 (estimate)
storage temp.
APPROX 4°C
pka
12.09±0.46(Predicted)
Water Solubility
1800 mg l-1 (20 °C)
form
neat
BRN
2973224
CAS DataBase Reference
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
RIDADR
2992
WGK Germany
3
RTECS
FC2628000
HazardClass
6.1(b)
PackingGroup
III
HS Code
29309090
Toxicity
LC50 (96-hour) for carp 10–20 mg/L, golden orfe 8–10 mg/L, goldfish 20–40 mg/L and rudd 10–20 mg/L (Hartley and Kidd, 1987); acute oral LD50 for rats 411–499 mg/kg (Hartley and Kidd, 1987), 200 mg/kg (RTECS, 1985).
▼
▲
ETHIOFENCARB Usage And Synthesis
▼
▲
Uses
Ethiofencarb is a kind of Systemic insecticide ,used to control aphids on fruit crops.
Uses
Ethiofencarb is a systemic insecticide with contact and stomach action. It is used to control aphids on fruit, vegetables, ornamentals and sugar beet.
Environmental Fate
Plant. Degrades in plants to the sulfone and sulfoxide (Hartley and Kidd, 1987).
Metabolic pathway
Ethiofencarb is metabolised by rapid oxidation at sulfur, hydrolysis of the carbamate group to give phenols, hydroxylation of the N-methyl moiety and conjugation.
Degradation
Ethiofencarb is stable in neutral and acidic but is hydrolysed under basic conditions. DT50 values at pH 7 and 11.4 (37 °C) were 450 hours and 5 minutes, respectively. The kinetics of hydrolysis of ethiofencarb in pure water and in aqueous solutions at pH 2,6,9,12 and at temperatures in the range 4-50°C were studied. No acid hydrolysis was observed. Ethiofencarb was rapidly hydrolysed at pH 9 and 12. Ethiofencarb in pure water at room temperature reached an equilibrium with 80% remaining undegraded (Sanz-Asensio et al., 1997).
Photodegradation of aqueous solutions in sunlight is rapid (PM). The oxidative photodegradation of ethofencarb was studied in aqueous acetonitrile using anthraquinone to mimic natural photosensitisers. Solutions were irradiated with a Hg lamp (400 W) for 48 minutes. The emission spectrum of the lamp was not described. Reaction products were identified by GC-MS methods. The main products were 2- hydroxybenzaldehyde (2) and 3-methylbenzo[e-1,3]oxazine-2,4-dione(3) (see Scheme 1). Products resulted from photocleavage of the CH,-S bond and/or the C-O bond followed by hydrogen atom abstraction and photo-oxidation. An electron-acceptor photosensitiser may increase rates of photodegradation (Galadi and Julliard, 1996). Solutions of ethiofencarb in cyclohexane, cyclohexene or isopropanol were irradiated with a high pressure Hg lamp (cut-off filter <280 nm) or natural sunlight (Germany, May-July). Analysis was by HPLC with diode-array detection, NMR, IR and MS. Half-lives of photodegradation were in the range 20 minutes to more than 20 hours. The predominant reaction (Scheme 1) was photo-oxidation of ethiofencarb to its sulfoxide (4). The cyclic dione (3) was a product of oxidation at the benzylic position. Ethiofencarb was photo-oxidised in cyclohexane to the sulfoxide (4) and the sulfone (5) and their corresponding phenols (8 and 9), the latter being a minor product. Subsequently the cyclised dione (3) was formed. In isopropanol, reaction with solvent gave addition products (6 and 7) and an unusual bis-diethylthio compound (10) (Kopf and Schwack, 1995).
Photodegradation of aqueous solutions in sunlight is rapid (PM). The oxidative photodegradation of ethofencarb was studied in aqueous acetonitrile using anthraquinone to mimic natural photosensitisers. Solutions were irradiated with a Hg lamp (400 W) for 48 minutes. The emission spectrum of the lamp was not described. Reaction products were identified by GC-MS methods. The main products were 2- hydroxybenzaldehyde (2) and 3-methylbenzo[e-1,3]oxazine-2,4-dione(3) (see Scheme 1). Products resulted from photocleavage of the CH,-S bond and/or the C-O bond followed by hydrogen atom abstraction and photo-oxidation. An electron-acceptor photosensitiser may increase rates of photodegradation (Galadi and Julliard, 1996). Solutions of ethiofencarb in cyclohexane, cyclohexene or isopropanol were irradiated with a high pressure Hg lamp (cut-off filter <280 nm) or natural sunlight (Germany, May-July). Analysis was by HPLC with diode-array detection, NMR, IR and MS. Half-lives of photodegradation were in the range 20 minutes to more than 20 hours. The predominant reaction (Scheme 1) was photo-oxidation of ethiofencarb to its sulfoxide (4). The cyclic dione (3) was a product of oxidation at the benzylic position. Ethiofencarb was photo-oxidised in cyclohexane to the sulfoxide (4) and the sulfone (5) and their corresponding phenols (8 and 9), the latter being a minor product. Subsequently the cyclised dione (3) was formed. In isopropanol, reaction with solvent gave addition products (6 and 7) and an unusual bis-diethylthio compound (10) (Kopf and Schwack, 1995).
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
