

MONURON
| Price | USD1.00 |
| Packge | 1ASSAYS |
- Min. Order:1ASSAYS
- Supply Ability:1ton
- Time:2020-01-01
Product Details
- Product NameMONURON
- CAS No.150-68-5
- EINECS No.200-154-8
- MFC9H11ClN2O
- MW198.65
- InChIKeyBMLIZLVNXIYGCK-UHFFFAOYSA-N
- AppearanceCrystalline SolidWhite
- Melting point 173-174 °C (lit.)
- storage temp. 0-6°C
- density 1,27 g/cm3
- Boiling point 301.31°C (rough estimate)
- Water Solubility 262mg/L(25 ºC)
▼
▲
MONURON Basic information
▼
▲
Product Name:
Synonyms:
3-(4-chlorophenoxy)-1,1-dimethylurea;3-(4-Chlorophenyl)-1,1-dimethylurea 6-methylphenol;3-(4-Chlor-phenyl)-1,1-dimethyl-harnstoff;3-(4-cloro-fenil)-1,1-dimetil-urea;3-(p-chlorophenyl)-1,1-dimethyl-ure;3-para-chlorophenyl-1,1-dimethylurea;chlorea;Chlorfenidim
CAS:
MF:
C9H11ClN2O
MW:
198.65
EINECS:
205-766-1
Product Categories:
Mol File:
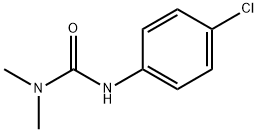
▼
▲
MONURON Chemical Properties
▼
▲
Melting point
173-174 °C(lit.)
Boiling point
301.31°C (rough estimate)
density
1,27 g/cm3
refractive index
1.5330 (estimate)
storage temp.
0-6°C
pka
14.22±0.70(Predicted)
form
Crystalline Solid
color
White
λmax
244nm(H2O)(lit.)
Merck
14,6261
BRN
2097922
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
RIDADR
UN 3077 9/PG 3
WGK Germany
3
RTECS
YS6300000
TSCA
Yes
HazardClass
9
PackingGroup
III
HS Code
29242990
Toxicity
LD50 orally in rats: 3700 mg/kg (Bailey, White)
▼
▲
MONURON Usage And Synthesis
▼
▲
Chemical Properties
White, crystalline solid; odorless. Very low solubility in water and hydrocarbonsolvents; slightly soluble in oils; partially soluble inalcohols; stable toward oxidation and moisture.
Uses
Herbicide, sugarcane-flowering suppressant.
Definition
ChEBI: A member of the class of ureas that is urea in which one of the nitrogens is substituted by a p-chlorophenyl group while the other is substituted by two methyl groups.
General Description
White crystalline solid or white powder with a slight odor. Melting point 175°C. Moderately toxic by ingestion. Used as an herbicide.
Air & Water Reactions
Insoluble in water. Is hydrolyzed slowly by acids and alkalis, and more rapidly on heating .
Reactivity Profile
MONURON is a chlorinated urea derivative. May react with azo and diazo compounds to generate toxic gases. May react with strong reducing agents to generate flammable gases. Reacts as a weak base. Combustion generates mixed oxides of nitrogen (NOx).
Hazard
Questionable carcinogen.
Health Hazard
Toxic properties are similar to Diuron; hydro-lyzes under acidic or alkaline conditions top-chloroaniline, which can cause anemia andmethemoglobinemia; LD50 data published inthe literature differ; acute and chronic tox-icity of this herbicide is probably of loworder; no reported case of human poisoning; showed clear evidence of carcinogenicity in male F344/N rats fed diets containing 750 ppm monuron for 2 years; causedcancers in the kidney and liver (NationalToxicology Program 1988); female rats andmale and female mice (B6C3F1) showed noevidence; induced cytomegaly of the renalepithelial cells in rats.
LD50 oral (rat): 3700 mg/kg (Bailey andWhite, 1965)
LD50 oral (rat): 1053 mg/kg (Lewis 1995).
LD50 oral (rat): 3700 mg/kg (Bailey andWhite, 1965)
LD50 oral (rat): 1053 mg/kg (Lewis 1995).
Fire Hazard
Flash point data for MONURON are not available; however, MONURON is probably combustible.
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and possibly other routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental carcinogenic data. Mutation data reported. An herbicide. When heated to decomposition it emits very toxic fumes of NOx and Cl-.
Environmental Fate
Biological. Monuron was mineralized in sewage samples obtained from a water treatment plant in Ithica, NY. (4-Chlorophenyl)urea and 4-chloroaniline were tentatively identified as metabolites (Wang et al., 1985).
Soil/Plant. In soils and plants, monuron is demethylated at the terminal nitrogen atom coupled with ring hydroxylation forming 3-(2-hydroxy-4-chlorophenyl)urea and 3-(3- hydroxy-4-chlorophenyl)urea (Hartley and Kidd, 1987). Walln?efer et al. (197
Photolytic. When an aqueous solution of monuron was exposed to sunlight or simulated sunlight, the major degradative pathways observed were the photooxidation and demethylation of the N-methyl groups (Crosby and Tang, 1969; Tanaka et al., 1982a),
Tanaka et al. (1981) studied the photolysis of monuron in dilute aqueous solutions in order to fully characterize a substituted diphenylamine that was observed in an earlier investigation (Tanaka et al., 1977). They identified this compound as an isomeric mixture containing 92% 2-chloro-4¢,5-bis(N¢,N¢-dimethylureido)biphenyl and 8% 5-chloro-2,4¢- bis(N¢,N¢-dimethylureido)biphenyl (Tanaka et al., 1981).
Tanaka et al. (1982) undertook a study to identify the several biphenyls formed in earlier photolysis studies (Tanaka et al., 1979, 1981). They identified these compounds as 2,4¢-, 3,4¢- and 4,4¢-bis-(N¢,N¢-dimethylureido)biphenyls (fenuron biphenyls) (Ta
Soil/Plant. In soils and plants, monuron is demethylated at the terminal nitrogen atom coupled with ring hydroxylation forming 3-(2-hydroxy-4-chlorophenyl)urea and 3-(3- hydroxy-4-chlorophenyl)urea (Hartley and Kidd, 1987). Walln?efer et al. (197
Photolytic. When an aqueous solution of monuron was exposed to sunlight or simulated sunlight, the major degradative pathways observed were the photooxidation and demethylation of the N-methyl groups (Crosby and Tang, 1969; Tanaka et al., 1982a),
Tanaka et al. (1981) studied the photolysis of monuron in dilute aqueous solutions in order to fully characterize a substituted diphenylamine that was observed in an earlier investigation (Tanaka et al., 1977). They identified this compound as an isomeric mixture containing 92% 2-chloro-4¢,5-bis(N¢,N¢-dimethylureido)biphenyl and 8% 5-chloro-2,4¢- bis(N¢,N¢-dimethylureido)biphenyl (Tanaka et al., 1981).
Tanaka et al. (1982) undertook a study to identify the several biphenyls formed in earlier photolysis studies (Tanaka et al., 1979, 1981). They identified these compounds as 2,4¢-, 3,4¢- and 4,4¢-bis-(N¢,N¢-dimethylureido)biphenyls (fenuron biphenyls) (Ta
Purification Methods
Crystallise monuron from MeOH. [Beilstein 12 IV 1191.]
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
