

LEAD CARBONATE
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2019-12-31
Product Details
- Product NameLEAD CARBONATE
- CAS No.598-63-0
- EINECS No.209-943-4
- MFCO3Pb
- MW267.21
- InChIKeyMFEVGQHCNVXMER-UHFFFAOYSA-L
- AppearancePowderWhite
- Melting point 399-401°C (dec.)
- density 6.6 g/cm3
- Water Solubility Soluble in acid and alkali. Insoluble in water, alcohol and ammonia.
- storage temp. Store below +30°C.
▼
▲
Product Name:
Synonyms:
LEAD CARBONATE, 99.999%LEAD CARBONATE, 99.999%LEAD CARBONATE, 99.999%LEAD CARBONATE, 99.999%;LEAD CARBONATE, REAGENT (ACS)LEAD CARBONATE, REAGENT (ACS)LEAD CARBONATE, REAGENT (ACS)LEAD CARBONATE, REAGENT (ACS);carbonicacid,lead(2++)salt(1:1);cerussete;dibasicleadcarbonate;lead(2+)carbonate;leadcarbonate(pbco3);Carbonic acid lead(II)
CAS:
MF:
CO3Pb
MW:
267.21
EINECS:
209-943-4
Product Categories:
Mol File:
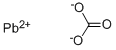
▼
▲
LEAD CARBONATE Chemical Properties
▼
▲
Melting point
399-401°C (dec.)
density
6.6 g/cm3
refractive index
2.08
storage temp.
Store below +30°C.
form
Powder
color
White
PH
5.8 (50g/l, H2O, 20℃)(slurry)
Water Solubility
Soluble in acid and alkali. Insoluble in water, alcohol and ammonia.
Stability:
Stable. Incompatible with strong acids, strong oxidizing agents.
CAS DataBase Reference
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
RIDADR
UN 3077 9/PG 3
WGK Germany
3
RTECS
OF9275000
TSCA
Yes
HS Code
2836 99 17
HazardClass
6.1
PackingGroup
III
▼
▲
▼
▲
LEAD CARBONATE Usage And Synthesis
▼
▲
Lead carbonate occurs in nature as the mineral cerussite. It has several applications. The compound is used in high pressure lubricating greases; as a coating on polyvinyl chloride to improve the dielectric properties of the polymers; in the PVC friction liners for pulleys; in corrosion-resistant grids in lead-storage batteries; in heat-sensitive sheets for thermographic copying; as a photoconductor in electrophotography; in thermistors; and in waxes for steel cables. Another major application of this compound is in catalysis—to catalyze polymerization of formaldehyde to high molecular weight polymeric products and to accelerate the process of curing of moldable thermosetting silicone resins.
Colorless orthorhombic crystals; refractive index 1.804; Moh’s hardness 3–3.5; density 6.60 g/cm3; decomposes on heating at 315°C; practically insoluble in water (1.1 mg/L at 20°C); KSP 1.46x10–13 at 25°C; also insoluble in alcohol and ammonia; soluble in acids and alkalies.
Lead carbonate is prepared by passing carbon dioxide into a cold dilute solution of lead acetate:
Pb(C2H3O2)2 + CO2 + H2O → PbCO3 + CH3COOH
The compound also is prepared in the laboratory by adding sodium bicarbonate to a cold dilute solution of a lead(II) salt, such as lead nitrate or acetate:
Pb2+ + 2HCO3¯ → PbCO3 + CO2 + H2O
Pb(C2H3O2)2 + CO2 + H2O → PbCO3 + CH3COOH
The compound also is prepared in the laboratory by adding sodium bicarbonate to a cold dilute solution of a lead(II) salt, such as lead nitrate or acetate:
Pb2+ + 2HCO3¯ → PbCO3 + CO2 + H2O
When heated at 315°C, lead carbonate decomposes to lead oxide and carbon dioxide:
PbCO3→PbO + CO2
When heated in water, it transforms to basic lead carbonate, 2PbCO3•Pb(OH)2
3PbCO3 + H2O → 2PbCO3•Pb(OH)2 + CO2
Lead carbonate dissolves in acids, forming the corresponding lead salt and evolving carbon dioxide:
PbCO3 + 2HCl → PbCl2 + H2O + CO2
Reaction with concentrated acetic acid yields anhydrous lead(II) acetate.
Fusion with boric acid at high temperature forms lead metaborate that has an approximate composition Pb(BO2)2•H2O. The product loses water of crystallization at 160°C.
PbCO3→PbO + CO2
When heated in water, it transforms to basic lead carbonate, 2PbCO3•Pb(OH)2
3PbCO3 + H2O → 2PbCO3•Pb(OH)2 + CO2
Lead carbonate dissolves in acids, forming the corresponding lead salt and evolving carbon dioxide:
PbCO3 + 2HCl → PbCl2 + H2O + CO2
Reaction with concentrated acetic acid yields anhydrous lead(II) acetate.
Fusion with boric acid at high temperature forms lead metaborate that has an approximate composition Pb(BO2)2•H2O. The product loses water of crystallization at 160°C.
Although an insoluble salt of lead, the compound exhibits low-to-moderate systemic effects from ingestion in humans. The effects are gastrointestinal contractions, jaundice, convulsions, nausea or vomiting, and degenerative changes in the brain (Lewis (Sr.), R. J. 1996. Sax’s Dangerous Properties of Industrial Materials, 9th ed. New York: Van Nostrand Reinhold).
Chemical Properties
colorless ortho-rhomb crystal(s); made by adding CO2 to a cold dilute solution of lead acetate; many uses such as a catalyst for organic reactions, in high temp greases, as a photoconductor in electrophotography [KIR78]
Uses
Preparation of lead standard solutions
Uses
Lead carbonate (PbCO3) is found in nature as cerussite. It can also be produced in the laboratory by reacting sodium carbonate with chlorine. It is a crystalline poison that was, and to a lesser extent still is, used as a pigment in white house paints.
Definition
A naturally occurring form of lead(II) carbonate that is an important lead ore. It forms orthorhombic crystals and is often found together with galena (PbS).
Definition
cerussite: An ore of lead consistingof lead carbonate, PbCO3. It is usuallyof secondary origin, formed by theweathering of galena. Pure cerussiteis white but the mineral may be grey due to the presence of impurities. Itforms well-shaped orthorhombiccrystals. It occurs in the USA, Spain,and SW Africa.
▼
▲
LEAD CARBONATE Preparation Products And Raw materials
▼
▲
Preparation Products
▼
▲
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- 598-63-0 LEAD (II) CARBONATE 99%
- Inquiry
- 2024-11-05
-
VIP1年
- Pallav Chemicals And Solvents Pvt Ltd
- 598-63-0 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Nithyasri Chemicals APURVA CHEMICALS
- 598-63-0 98%
- Inquiry
- 2024-01-24
-
VIP1年
- JSK Chemicals
- 598-63-0 Lead(II) carbonate, 98% 99%
- Inquiry
- 2023-12-22
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
