

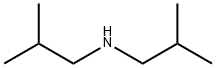
Diisobutylamine
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2019-12-31
Product Details
- Product NameDiisobutylamine
- CAS No.110-96-3
- EINECS No.203-819-3
- MFC8H19N
- MW129.24
- InChIKeyNJBCRXCAPCODGX-UHFFFAOYSA-N
- AppearanceLiquidClear
- Melting point -77 °C (lit.)
- Water Solubility 5 g/L (20 ºC)
- Boiling point 137-139 °C (lit.)
- density 0.74 g/mL at 25 °C (lit.)
- storage temp. Flammables area
▼
▲
Product Name:
Synonyms:
Diisobutylamin;diisobutylamine,[flammableliquid];N-(2-Methylpropyl)-2-methyl-1-propanamin;N-(2-Methylpropyl)-2-methyl-1-propanamine;N,N-Bis(2-methylpropyl)amine;N-Isobutyl-2-methyl-1-propanamine;2-METHYL-N-(2-METHYLPROPYL)-1-PROPANAMINE;AURORA KA-7633
CAS:
MF:
C8H19N
MW:
129.24
EINECS:
203-819-3
Product Categories:
Mol File:
▼
▲
Diisobutylamine Chemical Properties
▼
▲
Melting point
-77 °C
Boiling point
137-139 °C(lit.)
density
0.74 g/mL at 25 °C(lit.)
refractive index
n20/D 1.4081(lit.)
Fp
85 °F
storage temp.
Flammables area
solubility
insoluble to slightly soluble in water; soluble in ethanol, methanol, ethyl ether, ethyl acetate, acetone, benzene, aromatic and aliphatic hydrocarbons, fixed oils, mineral oil, oleic and stearic acids
pka
11.07±0.28(Predicted)
form
Liquid
color
Clear
Water Solubility
5 g/L (20 ºC)
Sensitive
Air Sensitive
BRN
1209251
CAS DataBase Reference
NIST Chemistry Reference
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
RIDADR
UN 2361 3/PG 3
WGK Germany
1
RTECS
TX1750000
F
TSCA
Yes
HazardClass
3
PackingGroup
III
HS Code
29211990
Hazardous Substances Data
▼
▲
▼
▲
Diisobutylamine Usage And Synthesis
▼
▲
Chemical Properties
CLEAR LIQUID
Chemical Properties
The chemical reactivity of diisobutylamine is similar to other aliphatic amines and is governed by the unshared electron pair on the nitrogen atom. It is a strong base tending to form salts with acids. As with other secondary amines, diisobutylamine can be nitrosated, especially under acidic conditions, by nitrite ion or by nitrogen oxides from the air to form the carcinogenic and mutagenic N-nitrosodiisobutylamine (Olah et al 1975).
Production Methods
Diisobutylamine can be produced by the reaction of ammonia and butanol over a dehydration catalyst at high temperature and pressure (Hawley 1977). Alternatively, ammonia, butanol, and hydrogen can be passed over a dehydrogenation catalyst. In 1976, 18,000 tons of diisobutylamine were produced (Schweizer et al 1978). Diisobutylamine is also naturally present in foods and soil.
As with other secondary amines, diisobutylamine can be nitrosated to form the highly toxic (Olah 1975) N-nitrosodiisobutylamine (Guttenplan 1987; Vlasenko et al 1981; Spiegeholder et al 1978). Thus, nitrosation of commercial preparations of diisobutylamine occurs on standing, presumably by reaction with nitrogen oxides in the air (Spiegelhalder et al 1978) and N-nitrosodiisobutylamine has been found in various fishery products (Kawabata et al 1974) and other foods (Osborne 1972; Telling 1972).
As with other secondary amines, diisobutylamine can be nitrosated to form the highly toxic (Olah 1975) N-nitrosodiisobutylamine (Guttenplan 1987; Vlasenko et al 1981; Spiegeholder et al 1978). Thus, nitrosation of commercial preparations of diisobutylamine occurs on standing, presumably by reaction with nitrogen oxides in the air (Spiegelhalder et al 1978) and N-nitrosodiisobutylamine has been found in various fishery products (Kawabata et al 1974) and other foods (Osborne 1972; Telling 1972).
General Description
A clear colorless liquid with an ammonia-like odor. Insoluble in water and less dense than water. Hence floats on water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.
Air & Water Reactions
Highly flammable. Sensitive to heat and air. Insoluble in water.
Reactivity Profile
Diisobutylamine can react vigorously with oxidizing materials . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
Inhalation of high concentrations of vapor will cause irritation of the respiratory tract and the lungs. Contact with liquid may result in severe skin and eye irritation. Exposure to concentrated vapors may result in corneal edema. Poisonous if swallowed.
Health Hazard
Diisobutylamine is a relatively strong base and is therefore irritating to the eyes, respiratory tract, and skin. Inhalation of vapors can result in pulmonary edema following prolonged exposure. Ingestion of liquid can cause severe burning of the esophagus.
Industrial uses
Diisobutylamine is used as a chemical intermediate in the manufacture of several agricultural and pharmaceutical products.
Safety Profile
Poison by ingestion. A dangerous fire hazard when exposed to heator flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of Nox
Metabolism
There is little information available on the metabolism of diisobutylamine.
▼
▲
Diisobutylamine Preparation Products And Raw materials
▼
▲
Preparation Products
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
