

Melamine
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2019-12-31
Product Details
- Product NameMelamine
- CAS No.108-78-1
- EINECS No.203-615-4
- MFC3H6N6
- MW126.12
- InChIKeyJDSHMPZPIAZGSV-UHFFFAOYSA-N
- AppearanceFine Crystalline PowderWhite
- storage temp. no restrictions.
- Melting point >300 °C (lit.)
- density 1.573
- Water Solubility 3 g/L (20 ºC)
- Boiling point 224.22°C (rough estimate)
▼
▲
Product Name:
Synonyms:
Melamine (Metformin EP Impurity D);Melamine (Metformin Impurity D);Melamine standard solution of substance;Metformin impurity D (Ph Eur);Imp. D:melamine;2,4,6-Triamino-1,3,5-triazine for synthesis;Aero;Cyanuric triamide
CAS:
MF:
C3H6N6
MW:
126.12
EINECS:
203-615-4
Product Categories:
Mol File:
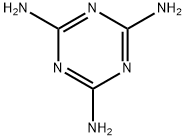
▼
▲
Melamine Chemical Properties
▼
▲
Melting point
354 °C
Boiling point
224.22°C (rough estimate)
density
1.573
vapor pressure
66.65 hPa (315 °C)
refractive index
1.872
Fp
>110°C
storage temp.
−20°C
solubility
water: soluble25mg/mL, clear to slightly hazy, colorless
pka
5(at 25℃)
form
Fine Crystalline Powder
color
White
PH
7-8 (32g/l, H2O, 20℃)
Water Solubility
3 g/L (20 ºC)
Merck
14,5811
BRN
124341
Stability:
Stable. Incompatible with strong acids, strong oxidizing agents. Nonflammable.
InChIKey
JDSHMPZPIAZGSV-UHFFFAOYSA-N
CAS DataBase Reference
NIST Chemistry Reference
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
RIDADR
3263
WGK Germany
1
RTECS
OS0700000
Autoignition Temperature
>600 °C
TSCA
Yes
PackingGroup
III
HS Code
29336980
Hazardous Substances Data
Toxicity
LD50 orally in Rabbit: 3161 mg/kg LD50 dermal Rabbit > 1000 mg/kg
▼
▲
▼
▲
Melamine Usage And Synthesis
▼
▲
Chemical Properties
White Solid
Chemical Properties
Melamine is a white crystalline solid
Uses
A compound that forms synthetic resins with formaldehyde
Uses
Forms synthetic resins with formaldehyde.
Uses
It is used to make high-pressure laminating resins (e.g., decorative countertops), molded compounds (e.g., dinnerware), and surface coating resins (e.g., appliance finishes and automotive topcoats). Additional major products are textile and paper treatment resins. Miscellaneous uses include adhesive resins for gluing lumber, plywood, and flooring, and resins for leather tanning agents. Melamine, melamine cyanurate, other melamine salts, and guanidine compounds are currently the most used group of nitrogencontaining flame retardants. Melamine is used as a flame retardant additive for polypropylene and polyethylene. Melamine cyanurate is employed commercially as a flame retardant for polyamides and terephthalates.
Definition
ChEBI: A trimer of cyanamide, with a 1,3,5-triazine skeleton.
Definition
A white solid organic compound whose molecules consist of a sixmembered heterocyclic ring of alternate carbon and nitrogen atoms with three amino groups attached to the carbons. Condensation polymerization with methanal or other aldehydes produces melamine resins, which are important thermosetting plastics.
Definition
melamine: A white crystalline compound,C3N6H6. Melamine is a cycliccompound having a six-memberedring of alternating C and N atoms,with three NH2 groups. It can becopolymerized with methanal to givethermosetting melamine resins,which are used particularly for laminatedcoatings.
Production Methods
Melamine is prepared almost exclusively by the urea process—the action of ammonia on urea. It is produced worldwide.
General Description
Colorless to white monoclinic crystals or prisms or white powder. Sublimes when gently heated.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Melamine is incompatible with strong oxidizing agents and strong acids . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Hazard
Toxic by ingestion, skin, and eye irritant. Questionable carcinogen.
Fire Hazard
Literature sources indicate that Melamine is nonflammable.
Contact allergens
Melamine-formaldehyde resin (MFR) results from condensation of melamine and formaldehyde. It is anactive ingredient of strong (reinforced) plasters, such as industrial or some dental plasters used for molding.It is also used as a textile finish resin. MFR acts as an allergen generally because of formaldehyde releasing (see Chap. 40)
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. An eye, skin, and mucous membrane irritant. Causes dermatitis in humans. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx and CN-.
Potential Exposure
Manufactured from urea, melamine is used in the manufacture of plastics, melamineformaldehyde resins; rubber, synthetic textiles; laminates, adhesives, and molding compound
Carcinogenicity
A bioassay of melamine was conducted in rats and mice by NTP. Male F344 rats and B6C3F1 mice were administered melamine in their diets at concentrations of 2250 or 4500 ppm daily for 103 weeks.Female rats were fed 4500 or 9000 ppm melamine. At the end of 111 weeks, surviving animals were killed and examined.
Purification Methods
Crystallise Melamine from water or dilute aqueous NaOH. It sublimes at ~240o on prolonged heating. [Beilstein 26 I 74, 26 II 132, 26 III/IV 1253.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Melamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents such as hydrides, nitrides, alkali metals, and sulfides.
▼
▲
Melamine Preparation Products And Raw materials
▼
▲
Preparation Products
PAINT-->Amino resin paint-->Various color amino baking enamel A04-9-->Boron nitride-->REACTIVE BLUE 4-->conductive coating-composite system of acrylic copolymer and cuprous iodide-->demulsifier KN-1-->MELAMINE RESIN-->Slushing agent,high efficiency-->Fluorescent Brightener BC-->Acid Red 92-->water proofing agent 703-->REACTIVE GREEN 19-->softener PEG-->Amino resin varnish-->Early-strength admixture-->Amino moulding plastic-->Pumping agent-->anchorage used for glassine paper-->modified phenol-formaldehyde resin-->Trimethylolmelamine Resin-->finishing agent KB for polyester viscose blend
Raw materials
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- MELAMINE 108-78-1 99%
- Inquiry
- 2024-11-05
-
VIP1年
- Pallav Chemicals And Solvents Pvt Ltd
- 108-78-1 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Orgamine Chemicals(I) Pvt Ltd
- 108-78-1 98%
- Inquiry
- 2024-03-01
-
VIP1年
- Gujarat State Fertilizers And Chemicals Ltd
- 108-78-1 Melamine 98%
- Inquiry
- 2024-02-27
-
VIP1年
- Varanous Labs Pvt Ltd
- Metformin EP Impurity D 108-78-1 98%
- Inquiry
- 2023-12-26
-
VIP1年
- UNILOSA INTERNATINAL PRIVATE LIMITED
- Melamine 99%
- Inquiry
- 2023-12-16
-
VIP1年
- Chem stride
- 108-78-1 99%
- Inquiry
- 2023-12-05
-
VIP1年
- Reenold Enterprises
- Melamine 99%
- Inquiry
- 2023-11-23
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
