

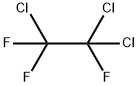
1,1,2-Trichlorotrifluoroethane
| Price | USD1.00 |
| Packge | 1IU |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2019-12-29
Product Details
- Product Name1,1,2-Trichlorotrifluoroethane
- CAS No.76-13-1
- EINECS No.200-936-1
- MFC2Cl3F3
- MW187.38
- InChIKeyAJDIZQLSFPQPEY-UHFFFAOYSA-N
- AppearanceColorless gas; volatile liquid.
- Boiling point 47-48 °C(lit.)
- Water Solubility 0.02 g/100 mL. Slightly soluble
- Melting point −35 °C(lit.)
- density 1.57 g/mL at 25 °C(lit.)
- storage temp. 2-8°C
▼
▲
Product Name:
Synonyms:
forane113;Freon 113 TR-T;Freon F113;Freon R 113;Freon TF;Freon TF (113);freon113tr-t;freonf113
CAS:
MF:
C2Cl3F3
MW:
187.38
EINECS:
200-936-1
Product Categories:
Mol File:
▼
▲
1,1,2-Trichlorotrifluoroethane Chemical Properties
▼
▲
Melting point
−35 °C(lit.)
Boiling point
47-48 °C(lit.)
density
1.57 g/mL at 25 °C(lit.)
vapor density
6.5 (vs air)
vapor pressure
5.5 psi ( 20 °C)
refractive index
n20/D 1.358(lit.)
Fp
195°C
storage temp.
2-8°C
solubility
0.17g/l
Water Solubility
0.02 g/100 mL. Slightly soluble
BRN
1740335
Henry's Law Constant
0.154, 0.215, 0.245, 0.319, and 0.321 at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth et al., 1988)
Exposure limits
TLV-TWA 1000 ppm (~7600 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 1250 ppm (ACGIH).
Stability:
Stable. Non-flammable. Incompatible with alkali metals, chemically active metals, magnesium, zinc, aluminium.
CAS DataBase Reference
NIST Chemistry Reference
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
RIDADR
UN 3082 9/PG 3
WGK Germany
2
RTECS
KJ4000000
Autoignition Temperature
1256 °F
Hazard Note
Irritant
Hazardous Substances Data
Toxicity
Acute oral LD50 for rats 43 mg/kg (quoted, RTECS, 1985).
▼
▲
Provider
Language
English
▼
▲
1,1,2-Trichlorotrifluoroethane Usage And Synthesis
▼
▲
Chemical Properties
colourless liquid or gas
Chemical Properties
TTE is a colorless liquid. Carbon tetrachloride-like odor at high concentrations. A gas above 48℃.
Physical properties
Clear, colorless liquid with a carbon tetrachloride-like odor at high concentrations
Uses
1,1,2-Trichloro-1,2,2-trifluoroethane is usedas a refrigerant and as a drycleaning solvent.It is also used as an extraction solvent foranalyzing petroleum hydrocarbons, oils, andgreases.
Uses
Dry-cleaning solvent, fire extinguishers, to make chlorotrifluoroethylene, blowing agent, polymer intermediate, solvent drying, drying electronic parts and precision equipment.
General Description
Colorless liquid with a sweet, ether-like odor. Sinks in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,1,2-Trichlorotrifluoroethane yields violent reactions with Al, Ba, Li, Sm, Na/K alloy and Ti . May react exothermically with aluminum.
Health Hazard
Inhalation causes irritation of the nose, throat, and lungs. High concentrations may cause death by respiratory failure or asphyxiation. May produce superficial skin burns or defatting type dermatitis and may irritate the eyes.
Health Hazard
The acute oral toxicity of this compoundis very low. The oral LD50 value in ratsis 43,000 mg/kg. The inhalation toxicity isalso low. Exposure to high concentrationscan produce a weak narcotic effect, car diac sensitization, and irritation of respi ratory passage. Chronic exposure causedliver enlargement in rats. A 6-hour expo sure to 87,000 ppm of 1,1,2-trichloro-1,2,2-trifluoroethane was lethal to rats. Ingestionof the liquid may cause nausea, lethargy,nervousness, and tremor. The irritant actionof the liquid was mild on rabbits’ skin.
Fire Hazard
Noncombustible liquid.
Industrial uses
1,1,2-Trichlorotrifluoroethane (CFC 113) is generally a stable molecule not prone to the reactivity that is often shown by the chlorinated hydrocarbons. The solvent blends that contain the fluorinated hydrocarbon and an alcohol display some metal reactivity which is inhibited by adding nitromethane as a stabilizer.
Safety Profile
Mildly toxic by ingestion and inhalation. Affects the central nervous system in humans. A sktn irritant. Combustible when exposed to heat or flame. Incompatible with Al, Ba, Li, Sm, NaK alloy, Ti. See also CHLORINATED HYDROCARBONS, ALIPHATIC; and FLUORIDES.
Potential Exposure
TTE is used as a solvent and refrigerant; it is used in fire extinguishers; as a blowing agent and as an intermediate in the production of chlorotrifluoroethylene monomer by reaction with zinc.
Carcinogenicity
A 2 year inhalation toxicity study/carcinogenicity study was conducted by Trochimowicz et al. in which groups of 100 male and 100 female rats were exposed to levels of up to of 20,000 ppm of CFC 113 6 h/day, 5 days/week for 2 years. Although five nasal tumors were seen, one at 20,000 ppm and four at 10,000 ppm, all five were different morphological types and were judged not to be exposure related. In addition, there was a small increase (5.8%) in pancreatic islet cell adenomas in the females exposed to 20,000 ppm; however, this was within the normal control range.
In a second study, injection of 0.1mLof 10% CFC 113 was not carcinogenic. But when given with a 5% solution of piperonyl butoxide, hepatomas were induced in male mice. The significance of this experimental finding has never been determined.
In a second study, injection of 0.1mLof 10% CFC 113 was not carcinogenic. But when given with a 5% solution of piperonyl butoxide, hepatomas were induced in male mice. The significance of this experimental finding has never been determined.
Environmental fate
Biological. In an anoxic aquifer beneath a landfill in Ottawa, Ontario, Canada, there was evidence to suggest that 1,1,2-trichlorotrifluoroethane underwent reductive dehalogenation to give 1,2-difluoro-1,1,2-trichloroethylene and 1,2-dichloro-1,1,2-trifluoroethane. It was proposed that the latter compound was degraded via dehydrodehalogenation to give 1-chloro-1,1,2-trifluoroethylene (Lesage et al., 1990).
Chemical/Physical. 1,1,2-Trichlorotrifluoroethane will not hydrolyze to any reasonable extent (Kollig, 1993).
Chemical/Physical. 1,1,2-Trichlorotrifluoroethane will not hydrolyze to any reasonable extent (Kollig, 1993).
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Wash it with water, then with weak alkali. Dry it with CaCl2 or H2SO4 and distil it. [Locke et al. J Am Chem Soc 56 1726 1934, Beilstein 1 III 157, 1 IV 142.]
Incompatibilities
Violent reaction with chemically active metals (such as powdered aluminum; beryllium, magnesium and zinc); calcium. Contact with alloys containing more than 2% magnesium causes decomposition, releasing hydrogen chloride, hydrogen fluoride; and carbon monoxide. May react exothermically with aluminum.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
▼
▲
1,1,2-Trichlorotrifluoroethane Preparation Products And Raw materials
▼
▲
Raw materials
Preparation Products
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
