





Factory high quality and low price LF163892 MONOHYDRATE
| Price | USD1.00 |
| Packge | 1g |
- Min. Order:1g
- Supply Ability:g/kg/Ton
- Time:2019-12-26
Product Details
- Product NameLF163892 MONOHYDRATE
- CAS No.76470-66-1
- EINECS No.617-954-4
- MFC16H16ClN3O4
- MW349.77
- AppearanceSolidWhite to Off-White
- storage temp. -20°C Freezer, Under inert atmosphere
- Melting point >196°C (dec.)
- density 1.52±0.1 g/cm3(Predicted)
- Boiling point 662.2±55.0 °C(Predicted)
Heidi 0706
Email:heidi@coreychem.com
▼
▲
LF163892 MONOHYDRATE Basic information
▼
▲
Product Name:
Synonyms:
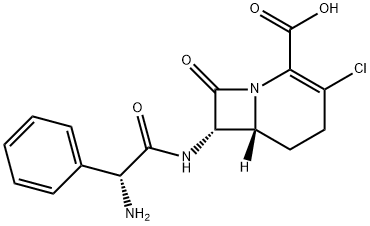
LORACARBEF MONOHYDRATE;LOEACARBEF;(6R)-7α-[[(R)-Aminophenylacetyl]amino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;(6R,6β)-7α-[[(R)-Aminophenylacetyl]amino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;3-Chloro-7β-[[(R)-aminophenylacetyl]amino]-1-carbacepham-3-ene-4-carboxylic acid;(6R,7S)-7-[[(2R)-2-AMino-2-phenylacetyl]aMino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid;Lorabid;1-Azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid, 7-[[(2R)-2-aMino-2-phenylacetyl]aMino]-3-chloro-8-oxo-, (6R,7S)-
CAS:
MF:
C16H18ClN3O5
MW:
367.78
EINECS:
Product Categories:
Mol File:
▼
▲
LF163892 MONOHYDRATE Chemical Properties
▼
▲
▼
▲
Safety Information
▼
▲
▼
▲
LF163892 MONOHYDRATE Usage And Synthesis
▼
▲
Uses
An antibiotic, a synthetic carbacephem analogue of cefaclor, and is more stable chemically.
Definition
ChEBI: A synthetic "carba" analogue of cefaclor, with carbon replacing sulfur at position 1. Used to treat a wide range of infections caused by both gram-positive and gram-negative bacteria.
Brand name
Lorabid (King).
Antimicrobial activity
An oral carbacephem, with carbon replacing sulfur in the fused ring structure. Its structure and properties are otherwise closely related to those of cefaclor, but it has improved chemical stability. Activity and stability to β-lactamases correspond closely to those of cefaclor.
It is almost completely absorbed by the oral route, but food delays absorption. A 500 mg oral dose achieves a serum concentration of around 16 mg/L after 1.3 h. Adequate concentrations are achieved for the treatment of upper respiratory tract infection. Sputum concentrations have been found to be around 2% of the corresponding plasma level. The plasma half-life is about 1 h and protein binding is 25%. Most of the dose is excreted unchanged in the urine, 60% within 12 h. The elimination half-life is increased in patients with impaired renal function. Probenecid delays excretion.
Diarrhea is the most prominent side effect, occurring in about 4% of patients. Other gastrointestinal upsets are also reported. It has been used for the oral treatment of upper respiratory tract infection, skin and soft-tissue infections, and uncomplicated urinary tract infection caused by sensitive organisms, but is not widely available.
It is almost completely absorbed by the oral route, but food delays absorption. A 500 mg oral dose achieves a serum concentration of around 16 mg/L after 1.3 h. Adequate concentrations are achieved for the treatment of upper respiratory tract infection. Sputum concentrations have been found to be around 2% of the corresponding plasma level. The plasma half-life is about 1 h and protein binding is 25%. Most of the dose is excreted unchanged in the urine, 60% within 12 h. The elimination half-life is increased in patients with impaired renal function. Probenecid delays excretion.
Diarrhea is the most prominent side effect, occurring in about 4% of patients. Other gastrointestinal upsets are also reported. It has been used for the oral treatment of upper respiratory tract infection, skin and soft-tissue infections, and uncomplicated urinary tract infection caused by sensitive organisms, but is not widely available.
Clinical Use
Loracarbef (Lorabid) is the first of a series of carbacephemsprepared by total synthesis to be introduced. Carbacephemsare isosteres of the cephalosporin (or △ 3-cephem) antibioticsin which the 1-sulfur atom has been replaced by a methylene(CH2) group. Loracarbef is isosteric with cefaclor and hassimilar pharmacokinetic and microbiological properties.Thus, the antibacterial spectrum of activity resembles thatof cefaclor, but it has somewhat greater potency againstH. influenzae and M. catarrhalis, including β-lactamase–producing strains. Unlike cefaclor, which undergoes degradationin human serum, loracarbef is chemically stable inplasma. It is absorbed well orally. Oral absorption is delayedby food. The half-life in plasma is about 1 hour.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
