





Factory high quality and low price NEOMYCIN SULFATE
| Price | USD1.00 |
| Packge | 1g |
- Min. Order:1g
- Supply Ability:g/kg/T
- Time:2019-12-25
Product Details
- Product NameNEOMYCIN SULFATE
- CAS No.1404-04-2
- EINECS No.215-766-3
- MFC23H52N6O25S3
- MW908.88
- Appearancepowder
- storage temp. 2-8°C
- Melting point 250 °C (decomp)
Heidi 0589
Email:heidi@coreychem.com
▼
▲
NEOMYCIN SULFATE Basic information
▼
▲
Product Name:
Synonyms:
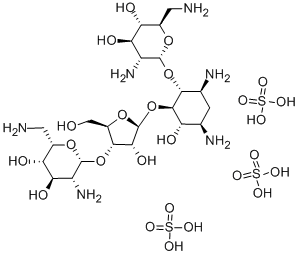
o-2,6-diamino-2,6-dideoxy-.beta.-l-idopyranosyl-(1.->3)-o-.beta.-d-ribofuranosyl-(1->5)]-o-[2,6-diamino-2,6-dideoxy-.alpha.-d-glucopyranosyl-(1->4)]-2-deoxy sulfate;NEOMYCIN;fradiomycin;myacyne;mycifradin;neolate;neomas;neomcin
CAS:
MF:
C23H52N6O25S3
MW:
908.88
EINECS:
215-766-3
Product Categories:
Mol File:
▼
▲
NEOMYCIN SULFATE Chemical Properties
▼
▲
Melting point
250 °C (decomp)
storage temp.
2-8°C
solubility
H2O: 50 mg/mL As a stock solution. Stock solutions should be filter sterilized and stored at 2-8°C. Stable at 37°C for 5 days.
form
powder
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
WGK Germany
3
RTECS
QP4375000
F
Toxicity
LD50 oral in rat: 2750mg/kg
▼
▲
NEOMYCIN SULFATE Usage And Synthesis
▼
▲
Uses
Antibacterial; antifungal.
Definition
An antibiotic complex obtained from Streptomyces fradiae; it is soluble in water and methanol but insoluble in most organic solvents. It consists of three component substances, all of which function as antiinfective agents; some derivatives have fungicidal properties. The three types are A (also called neamine): C12H26N4O6; B: C 23H46N6O13 (also available as hydrochloride and sulfate); and C: C23H46O13
Brand name
Mycifradin (Pharmacia & Upjohn); Neo-Fradin (X Gen); Neobiotic (Pfizer).
Antimicrobial activity
Among other organisms susceptible in vitro (MIC 4–8 mg/L) are Pasteurella, Vibrio, Borrelia and Leptospira spp. It is active against M. tuberculosis, including streptomycin-resistant strains. Synergy has been reported with polymyxin B. The bactericidal effect is enhanced at alkaline pH.
Acquired resistance
Resistance is acquired in a stepwise fashion and staphylococci may become resistant as a result of prolonged topical use. The use of neomycin–bacitracin–polymyxin mixtures may contribute to this, as many strains resistant to neomycin are also resistant to bacitracin. Resistant enterobacteria may appear in the feces of patients treated orally and in those treated for prolonged periods; most have been found to possess multiple transferable antibiotic resistance. Cross-resistance with kanamycin is often due to the synthesis of APH(3′), although AAC(6′) some forms of AAC(3) and ANT(4′) also modify both neomycin and kanamycin. Resistant strains of Staph. aureus are usually more resistant to kanamycin than to neomycin. The rare enzyme AAC(1) confers resistance to neomycin and paromomycin, but not to other aminoglycosides.
Contact allergens
Neomycin is an antibiotic complex of the aminoglycosides group, extracted from Streptomyces fradiae. It is composed of neomycin A (neamin) and an isomer neobiosamin, either neomycin B (framycetin or Soframycin?) or neomycin C. Its use has been progressively forbidden in cosmetics and as an additive for animal feed. Occupational contact dermatitis occurs in workers at animal feed mills, in veterinaries, or in health workers. Nonoccupational dermatitis mainly concerns patients with chronic dermatitis, leg ulcers, or chronic otitis. Cross-sensitivity is usual with other aminoglycosides (amikacin, arbekacin, butirosin, dibekacin, gentamicin, isepamicin, kanamycin, paromomycin, ribostamycin, sisomycin, tobramycin), is rare with netilmicin and streptomycin, but nonexistent with spectinomycin.
Pharmacokinetics
Cmax 0.5 g intramuscular: 20 mg/L after 1 h
Plasma half-life: 2–3 h
Volume of distribution: 0.25–0.35 L/kg
Plasma protein binding: Low
Very little is absorbed after oral administration and more than 95% is eliminated unchanged in the feces. Peak plasma concentrations of less than 4 mg/L have been found after an oral dose of 3 g. Distribution and excretion resemble that of streptomycin, but the toxicity of neomycin precludes systemic administration except in the most extreme cases.
Plasma half-life: 2–3 h
Volume of distribution: 0.25–0.35 L/kg
Plasma protein binding: Low
Very little is absorbed after oral administration and more than 95% is eliminated unchanged in the feces. Peak plasma concentrations of less than 4 mg/L have been found after an oral dose of 3 g. Distribution and excretion resemble that of streptomycin, but the toxicity of neomycin precludes systemic administration except in the most extreme cases.
Clinical Use
Superficial infections with staphylococci and Gram-negative bacilli (topical; alone or in combination with bacitracin, chlorhexidine or polymyxin)
Treatment of staphylococcal nasal carriers (topical, in combination with chlorhexidine or bacitracin)
Eye infections (topical; alone or in combination)
Otitis externa (alone or with a corticosteroid)
Gut decontamination before abdominal surgery (oral)
Prophylaxis after urinary tract instrumentation (instillation)
Use is discouraged because of the possibility of promoting the appearance of aminoglycoside-resistant strains, and because of the risk of absorption with the consequent danger of systemic toxicity or neuromuscular blockade.
Treatment of staphylococcal nasal carriers (topical, in combination with chlorhexidine or bacitracin)
Eye infections (topical; alone or in combination)
Otitis externa (alone or with a corticosteroid)
Gut decontamination before abdominal surgery (oral)
Prophylaxis after urinary tract instrumentation (instillation)
Use is discouraged because of the possibility of promoting the appearance of aminoglycoside-resistant strains, and because of the risk of absorption with the consequent danger of systemic toxicity or neuromuscular blockade.
Side effects
Neomycin is the most likely of all the aminoglycosides to damage the kidneys and the auditory branch of the eighth nerve. This has almost entirely restricted it to topical and oral use.
Irreversible deafness may develop even if the drug is stopped at the first sign of damage. Loss of hearing may occur as a result of topical applications to wounds or other denuded areas, particularly if renal excretion is impaired. Instillation of ear drops containing neomycin can result in deafness. This generally develops in the second week of treatment and is usually reversible.
Rashes have been described in 6–8% of patients treated topically and these patients may be rendered allergic to other aminoglycosides. Nausea and protracted diarrhea may follow oral administration. Sufficient drug may be absorbed from the gut on prolonged oral administration to produce deafness but not renal damage. Intestinal malabsorption and superinfection have been seen in patients receiving 4–9 g per day and may develop in patients receiving as little as 3 g of the drug per day. Precipitation of bile salts by the drug may impair the hydrolysis of long-chain triglycerides. Large doses instilled into the peritoneal cavity at operation may be absorbed, with resultant systemic toxicity, and patients concurrently exposed to anesthetics and muscle relaxants are liable to suffer neuromuscular blockade, which is reversible by neostigmine.
Irreversible deafness may develop even if the drug is stopped at the first sign of damage. Loss of hearing may occur as a result of topical applications to wounds or other denuded areas, particularly if renal excretion is impaired. Instillation of ear drops containing neomycin can result in deafness. This generally develops in the second week of treatment and is usually reversible.
Rashes have been described in 6–8% of patients treated topically and these patients may be rendered allergic to other aminoglycosides. Nausea and protracted diarrhea may follow oral administration. Sufficient drug may be absorbed from the gut on prolonged oral administration to produce deafness but not renal damage. Intestinal malabsorption and superinfection have been seen in patients receiving 4–9 g per day and may develop in patients receiving as little as 3 g of the drug per day. Precipitation of bile salts by the drug may impair the hydrolysis of long-chain triglycerides. Large doses instilled into the peritoneal cavity at operation may be absorbed, with resultant systemic toxicity, and patients concurrently exposed to anesthetics and muscle relaxants are liable to suffer neuromuscular blockade, which is reversible by neostigmine.
Safety Profile
Poison by intraperitoneal, intravenous, and subcutaneous routes. Moderately toxic by ingestion. Human systemic effects: changes in hearing acuity, liver tubule changes, and decreased urine volume or anuria. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
Recommended supplier
-
VIP1年
- Triveni Interchem Private Limited
- 1404-04-2 Neomycin 98%
- Inquiry
- 2024-01-23
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
