

MANGANESE(II) ACETATE
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2019-12-20
Product Details
- Product NameMANGANESE(II) ACETATE
- CAS No.638-38-0
- EINECS No.211-334-3
- MFC4H6MnO4
- MW173.03
- InChIKeyUOGMEBQRZBEZQT-UHFFFAOYSA-L
- AppearancePowderGrayish-beige to light brown
- density 1.589
- Melting point 180°C
- Water Solubility Soluble in water, methanol and acetic acid.
- storage temp. Store below +30°C.
▼
▲
Product Name:
Synonyms:
octanmanganaty(czech);MANGANESE ACETATE;MANGANESE (II) 2,4-PENTANEDIONATE;MANGANESE(II) ACETATE;Manganeseacetateanhydrous;ManganeseAcetateTetrahydrateExtraPure;Manganese(II) acetate, anhydrous, 98+%;manganese(ii) acetate, anhydrous
CAS:
MF:
C4H6MnO4
MW:
173.03
EINECS:
211-334-3
Product Categories:
Mol File:
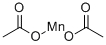
▼
▲
MANGANESE(II) ACETATE Chemical Properties
▼
▲
Melting point
180°C
density
1.589
storage temp.
Store below +30°C.
form
Powder
color
Grayish-beige to light brown
Water Solubility
Soluble in water, methanol and acetic acid.
Sensitive
Hygroscopic
Merck
13,5746
InChIKey
NKKRYPIWDQMINN-UHFFFAOYSA-M
CAS DataBase Reference
EPA Substance Registry System
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
WGK Germany
3
RTECS
AI5770000
TSCA
Yes
HS Code
2915 29 00
▼
▲
▼
▲
MANGANESE(II) ACETATE Usage And Synthesis
▼
▲
Description
Manganese (II) acetate is the chemical compound with the formula Mn(CH3COO)2. It is used as a desiccant, a catalyst, and as fertilizer.
Chemical Properties
Pale-red crystals.Soluble in water and alcohol. Combustible.
Uses
Textile dyeing, oxidation catalyst, paint and varnish (drier, boiled oil manufacture), fertilizers, food packaging, feed additive.
Reactions
Manganese (II) acetate can be formed by reacting acetic acid with either manganese (II,III) oxide or manganese (II) carbonate :
Mn3O4 + 2CH3COOH → Mn(CH3COO)2 + Mn2O3+ H2O
If manganese (II,III) oxide is used , manganese (III) oxide is produced as a byproduct.
If the anhydrous form needs to be produced, manganese (II) nitrate can be reacted with acetic anhydride.
Mn3O4 + 2CH3COOH → Mn(CH3COO)2 + Mn2O3+ H2O
If manganese (II,III) oxide is used , manganese (III) oxide is produced as a byproduct.
If the anhydrous form needs to be produced, manganese (II) nitrate can be reacted with acetic anhydride.
Purification Methods
Crystallise it from water acidified with acetic acid. [Beilstein 2 IV 120.]
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
Recommended supplier
-
VIP1年
- Monopoly Innovations Pvt. Ltd.
- 638-38-0 Manganese(II) acetate 98%
- Inquiry
- 2024-03-16
-
VIP1年
- Vinipul Chemicals Pvt Ltd
- 638-38-0 Manganese(II) acetate 99%
- Inquiry
- 2024-03-06
-
VIP1年
- Kezi Industries (Sam Industries)
- Manganese(II) acetate 638-38-0 98%
- Inquiry
- 2024-03-02
-
VIP1年
- Eastmen Chemicals
- Manganese(II) acetate 98%
- Inquiry
- 2024-02-07
-
VIP1年
- Cosmic Chemicals
- 638-38-0 98%
- Inquiry
- 2024-02-01
-
VIP1年
- Nithyasri Chemicals APURVA CHEMICALS
- 638-38-0 Manganese(II) acetate 98%
- Inquiry
- 2024-01-24
-
VIP1年
- Supra Group of Companies
- Manganese(II) acetate 638-38-0 99%
- Inquiry
- 2024-01-22
-
VIP1年
- Satyam Pharma Chem Pvt Ltd
- Manganese(II) acetate 99%
- Inquiry
- 2024-01-17
-
VIP1年
- Salicylates and Chemicals Pvt. Ltd.
- 638-38-0 98%
- Inquiry
- 2024-01-08
-
VIP1年
- allipo chemicals
- 638-38-0 Manganese Acetate Tetrahydrate 97%
- Inquiry
- 2023-11-22
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
