

Cefepime
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:1ton
- Time:2019-12-20
Product Details
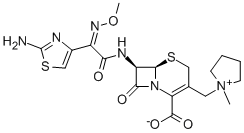
- Product Name Cefepime
- CAS No.88040-23-7
- EINECS No.200-110-4
- MFC19H24N6O5S2
- MW480.56
- AppearanceSolidWhite to light yellow
- Melting point 150 C
▼
▲
Cefepime Basic information
▼
▲
Product Name:
Synonyms:
[6R-[(6α,7β(Z)]]-7-[[(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-y1]methy1]-1-methylpyrrolidinium inner salt;(6R)-7α-[[(2-Amino-4-thiazolyl)[(Z)-methoxyimino]acetyl]amino]-3-[(1-methylpyrrolidinio)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid ion;(6R,6β)-7α-[[(2-Amino-4-thiazolyl)[(Z)-methoxyimino]acetyl]amino]-8-oxo-3-[[(1-methylpyrrolidinium)-1-yl]methyl]-5-thia-1-azabicyclo[4.2.0]octa-2-ene-2-carboxylic acidion;Cefpim;CFPM;PyrrolidiniuM, 1-[[(6R,7R)-7-[[(2Z)-2-(2-aMino-4-thiazolyl)-2-(MethoxyiMino)acetyl]aMino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]Methyl]-1-Methyl-, inner salt;Cefepime sodium;Cefepime impurity
CAS:
MF:
C19H24N6O5S2
MW:
480.56
EINECS:
643-019-5
Product Categories:
Mol File:
▼
▲
Cefepime Chemical Properties
▼
▲
Melting point
150 C
CAS DataBase Reference
▼
▲
Safety Information
▼
▲
▼
▲
Cefepime Usage And Synthesis
▼
▲
Maxipime
Description
Cefepime is a new fourth-generation parenteral cephalosporine antibiotic launched in 1993 in Sweden and France. Cefepime has broad spectrum antimicrobial activity against Staphylococcus, Strepfococcus, Pseudomonas, and the Enterobacteriaceae, including many bacterial isolates that are resistant to commonly used ceftazidime and cefotaxime. Its efficacy has been demonstrated in the treatment of lower respiratory tract infections especially pneumonia, intra-abdominal and urinary tract infections, skin and soft tissue infections, chronic osteomyelitis and in prophylaxis of biliary tract and prostate infections. It is well tolerated by patients and is reported to exhibit no significant drug interactions.
Chemical Properties
colorless Powder
Originator
Bristol-Myers Squibb (U.S.A.)
Brand name
Maxipime; Axepim
Antimicrobial activity
Its activity against common pathogens is comparable to that of group 4 cephalosporins, but it is somewhat more active against Ps. aeruginosa. Like cefpirome it has low affinity for the molecular class C cephalosporinases of many Gram-negative rods and is consequently active against most strains of Citrobacter spp., Enterobacter spp., Serratia spp. and Ps. aeruginosa that are resistant to cefotaxime and ceftazidime. It has poor activity against L. monocytogenes and against anaerobic organisms.
Pharmacokinetics
Cmax 2 g intravenous (30-min infusion): c. 160 mg/L end infusion
Plasma half-life: c. 2 h
Volume of distribution: 14–20 L
Plasma protein binding: 10–19%
It is well distributed. Penetration into tissues, including lung, appears to be similar to that of other aminothiazoyl cephalosporins. Very low concentrations are achieved in CSF in the absence of meningeal inflammation. It is secreted in breast milk.
It is partially metabolized, but 85% of the dose is excreted unchanged in the urine, achieving a concentration approaching 1 g/L within 4 h of a 1 g intravenous dose. Dosage adjustment is required in patients with impaired renal function, but hepatic impairment does not affect the pharmacokinetic properties.
Plasma half-life: c. 2 h
Volume of distribution: 14–20 L
Plasma protein binding: 10–19%
It is well distributed. Penetration into tissues, including lung, appears to be similar to that of other aminothiazoyl cephalosporins. Very low concentrations are achieved in CSF in the absence of meningeal inflammation. It is secreted in breast milk.
It is partially metabolized, but 85% of the dose is excreted unchanged in the urine, achieving a concentration approaching 1 g/L within 4 h of a 1 g intravenous dose. Dosage adjustment is required in patients with impaired renal function, but hepatic impairment does not affect the pharmacokinetic properties.
Clinical Use
Cefepime (Maxipime, Axepin) is a parenteral, β-lactamase–resistant cephalosporin that is chemically and microbiologicallysimilar to cefpirome. It also has a broadantibacterial spectrum, with significant activity against bothGram-positive and Gram-negative bacteria, including streptococci,staphylococci, Pseudomonas spp., and theEnterobacteriaceae. It is active against some bacterial isolatesthat are resistant to ceftazidime. The efficacy of cefepimehas been demonstrated in the treatment of urinary tract infections,lower respiratory tract infections, skin and soft tissueinfections, chronic osteomyelitis, and intra-abdominal andbiliary infections. It is excreted in the urine with a half-life of2.1 hours. It is bound minimally to plasma proteins. Cefepimeis also a fourth-generation cephalosporin.
Clinical Use
Proprietary name: Maxipime.
Preparation: Injection.
Dosage: Adult, i.m., i.v., 1–6 g per day in 2–3 divided doses.
Available in USA, most of Europe and Japan; not available in the UK.
Preparation: Injection.
Dosage: Adult, i.m., i.v., 1–6 g per day in 2–3 divided doses.
Available in USA, most of Europe and Japan; not available in the UK.
Side effects
It is used in the treatment of serious infections, particularly those in which resistant Gram-negative pathogens are known or suspected to be involved.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
Recommended supplier
-
VIP1年
- Concept Pharmaceuticals Ltd
- Cefepime 99%
- Inquiry
- 2024-03-07
-
VIP1年
- PROTECH TELELINKS
- 88040-23-7 98%
- Inquiry
- 2024-02-09
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
