

FUMAGILLIN
| Price | USD3.00 |
| Packge | 3KG |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2019-08-07
Product Details
- Product NameFUMAGILLIN
- CAS No.23110-15-8
- EINECS No.245-433-8
- MFC26H34O7
- MW458.54
- Appearancepowderwhite
- storage temp. 2-8°C
- Melting point 194-195℃
- Boiling point 484.03°C (rough estimate)
- density 1.1368 (rough estimate)
▼
▲
FUMAGILLIN Basic information
▼
▲
Product Name:
Synonyms:
fugilin;fumidil;h-3;l)oxiranyl]-1-oxaspiro[2.5]oct-6-yl]ester,[3theta-[3alpha,4alpha(2theta,3;FUGILLIN;FUMADIL B;FUMAGILLIN;FUMAGILLIN, ASPERGILLUS FUMIGATUS
CAS:
MF:
C26H34O7
MW:
458.54
EINECS:
245-433-8
Product Categories:
Mol File:
▼
▲
FUMAGILLIN Chemical Properties
▼
▲
Melting point
194-195℃
alpha
D25 -26.6° (c = 1 in 95% ethanol)
Boiling point
484.03°C (rough estimate)
density
1.1368 (rough estimate)
refractive index
1.5800 (estimate)
Fp
2℃
storage temp.
−20°C
solubility
ethanol: 1 mg/mL
form
powder
color
white
Sensitive
Air Sensitive
InChIKey
NGGMYCMLYOUNGM-ZILXAVPASA-N
▼
▲
Safety Information
▼
▲
Hazard Codes
Risk Statements
Safety Statements
RIDADR
UN 1648 3 / PGII
WGK Germany
3
RTECS
HE1750000
HS Code
29419090
Toxicity
LD50 in mice (mg/kg): ~800 s.c. (DiPaolo)
▼
▲
English
▼
▲
FUMAGILLIN Usage And Synthesis
▼
▲
Fumagillin is an antibiotic derived from the fungus Aspergillus fumigatus. It has widely been used both in human and veterinary medicine. It has appeared to be the most effective medicine in supressing cryptosporidiosis and microsporidiosis caused by Enterocytozoon bieneusi which can be fatal in HIV-infected persons[1, 2]. Due to its antiparasitic efficacy fumagillin has also been widely applied in veterinary medicine against microsporidiosis of bees and fish[3-5]. Fumagillin is very stable in honey[6] even at higher temperatures; for example it was detectable after having been kept at 80oC for 35 days[7]. Mixed with syrup it is very effective in supressing Nosema in hibernating honeybee colonies. However, it has not been effective against dormant spores of Nosema apis, thus has never eliminated the disease in the bee colony entirely.
Fumagillin has also been used in the treatment of microsporidiosis in fish including the ones caused by Myxobolus cerebralis[4,8] and Tetracapsuloides bryosalmona[9-11]. It is effective in curing microsporidial keratoconjunctivitis[12, 13]. Taken in orally it has appeared highly effective against chronic Enterocytozoon bieneusi infections in patients with AIDS and other types of immunodeficiencies[14]. For further administration, however, appropriate therapeutical models should be completely improved and side effects of the treatment should be eliminated[15].
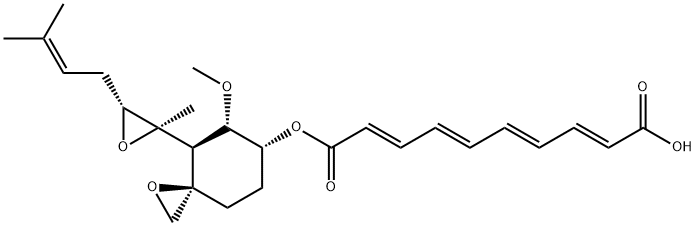
Figure 1 the chemical structure of fumagillin;
Fumagillin has also been used in the treatment of microsporidiosis in fish including the ones caused by Myxobolus cerebralis[4,8] and Tetracapsuloides bryosalmona[9-11]. It is effective in curing microsporidial keratoconjunctivitis[12, 13]. Taken in orally it has appeared highly effective against chronic Enterocytozoon bieneusi infections in patients with AIDS and other types of immunodeficiencies[14]. For further administration, however, appropriate therapeutical models should be completely improved and side effects of the treatment should be eliminated[15].

Figure 1 the chemical structure of fumagillin;
Fumagillin is applied against microsporidian infections and diseases in apiculture and in human medicine. Nosema disease is one of the most prevalent diseases encountered in apiculture1 and is now known to be caused by two species of single-cellular microsporidian parasites, N. apis and N. ceranae[16]. The phylum Microsporidia is composed of more than 160 genera and about 1300 different species[17, 18]. On the basis of molecular evidence, microsporidia are now considered to be highly specialized parasitic fungi[19, 20]. N. ceranae infects the bee digestive tract midgut epithelial cells[ventriculus] of adult workers and queens[21]. Similar to N. apis, the mature spores burst forth by rupturing the epithelial cells and spill into the midgut lumen, followed by defecation with the fecal matter. Infection spreads via a fecal−oral route when adult worker bees contract the infection by cleaning up fecal material originating from infected bees or through trophallaxis of contaminated food[22, 23]. Infection with N. ceranae has been shown to increase precocious foraging in worker bees, resulting in reduced life expectancy by 9 days on average in cage trials as compared with control groups[24]. Although outward signs of Nosema spp. infection cannot typically be seen, the inhibition of pollen digestion caused by the parasites leads to poor nourishment, smaller populations, reduced honey production, and higher winter colony mortality[25].
Fumagillin is a naturally occurring antibiotic compound that was first isolated in 1949 from an Aspergillus species, designated H-346 and later identified as Aspergillus fumigatus[26]. The drug was also discovered to be a potent amebicide[27]. The structure of fumagillin was eventually elucidated[28] through an extensive series of chemical manipulations, including the hydrolysis of fumagillin to yield the alcohol fumagillol. The importance of fumagillin as a treatment against the microsporidian fungal disease N. apis plaguing the European honey bee[A. mellifera L.] was soon recognized[29]. Fumagillin is also currently the only effective drug treatment available against N. ceranae[30, 31]. The commercial formulation of fumagillin consists of the dicyclohexylamine[DCH] salt of fumagillin. Fumagillin was also tested for the treatment of microsporidian infections in fish[32].
In human medicine, fumagillin is used as an inhibitor of microsporidian infections in patients with compromised immune systems due to acquired immunodeficiency syndrome(AIDS) or to relieve symptoms of intestinal microsporidiosis that occur after organ transplant procedures[33, 34]. More interestingly, though, fumagillin and its analogues are used to treat various cancers by inhibiting the formation of new blood vessels around growing tumors(angiogenesis), thereby limiting their blood supply[35].
Fumagillin is a naturally occurring antibiotic compound that was first isolated in 1949 from an Aspergillus species, designated H-346 and later identified as Aspergillus fumigatus[26]. The drug was also discovered to be a potent amebicide[27]. The structure of fumagillin was eventually elucidated[28] through an extensive series of chemical manipulations, including the hydrolysis of fumagillin to yield the alcohol fumagillol. The importance of fumagillin as a treatment against the microsporidian fungal disease N. apis plaguing the European honey bee[A. mellifera L.] was soon recognized[29]. Fumagillin is also currently the only effective drug treatment available against N. ceranae[30, 31]. The commercial formulation of fumagillin consists of the dicyclohexylamine[DCH] salt of fumagillin. Fumagillin was also tested for the treatment of microsporidian infections in fish[32].
In human medicine, fumagillin is used as an inhibitor of microsporidian infections in patients with compromised immune systems due to acquired immunodeficiency syndrome(AIDS) or to relieve symptoms of intestinal microsporidiosis that occur after organ transplant procedures[33, 34]. More interestingly, though, fumagillin and its analogues are used to treat various cancers by inhibiting the formation of new blood vessels around growing tumors(angiogenesis), thereby limiting their blood supply[35].
Fumagillin binds only to the MetAP-2 enzyme via the epoxide group located on the cyclohexane core ring structure and not to the MetAP-1 enzyme[36]. The other remaining epoxide on the molecule is not crucial for the binding to take place and is therefore dispensable.
MetAP-2 enzymes are found ubiquitously in all organisms[37]. Fumagillin acts against microsporidian as well as mammalian MetAP-2 enzyme, and the low selectivity of fumagillin between human and microsporidian MetAP-2 is the cause of its toxicity to humans as it also inhibits the human MetAP-2 enzyme necessary in protein maturation and post-translation processes[38]. A similar observation was recently reported whereby it was shown that fumagillin is active against honey-bee MetAP2 at low concentrations, whereas it has no therapeutic activity at those concentrations against N. ceranae MetAP-2[38].
MetAP-2 enzymes are found ubiquitously in all organisms[37]. Fumagillin acts against microsporidian as well as mammalian MetAP-2 enzyme, and the low selectivity of fumagillin between human and microsporidian MetAP-2 is the cause of its toxicity to humans as it also inhibits the human MetAP-2 enzyme necessary in protein maturation and post-translation processes[38]. A similar observation was recently reported whereby it was shown that fumagillin is active against honey-bee MetAP2 at low concentrations, whereas it has no therapeutic activity at those concentrations against N. ceranae MetAP-2[38].
Although fumagillin is an extremely beneficial compound in human medicine and in apiculture, some undesirable side effects cannot be ignored[33-35], Extended usage of fumagillin over prolonged periods of time, as required by chemotherapy, caused severe body weight loss of >15% from the starting weight in human test subjects[40]. In 1952, it was reported that fumagillin was essentially nontoxic to humans at oral doses of up to 50 mg daily for durations of 2 weeks to treat intestinal amebiasis[39]. Although no weight loss was observed in test subjects. A more recent study administered fumagillin orally up to 60 mg daily for 2 weeks to treat microsporidiosis in patients with HIV infection[33]. The authors acknowledged significant bone marrow toxicity of fumagillin with 4 patients of a group of 11 developing severe toxic side effects at the highest dosage administered (60 mg). These effects ceased within days of the treatment being terminated.
Common side effects in human trials in which fumagillin is administered orally are gastrointestinal-related cramping, diarrhea, and significant loss of body weight[40]. This undesirable weight loss side effect prompted recent, perhaps ethically questionable, trial use of fumagillin as a chemical mitigation for obesity[41].
Fumagillin was observed to exhibit significant negative chromosomal aberration effects at 50−75 mg/kg body weight in mice. Concentrations of 25, 50, and 75 mg/kg body weight were administered by gavage[42]. All experimental dosages listed above induced significant antiproliferative and genotoxic potential in mice[43]. Fumagillin exhibited clastogenic activity in human lymphocytes at concentrations equivalent to the therapeutic dose in beekeeping[44]. Genotoxicity to mouse bone marrow cells at concentrations of 10−20 mg/kg body weight administered in vivo to mice by gastric probe (5, 10, and 20 mg/kg concentrations tested), as compared to a control group, was also observed[45]. These results were confirmed with fumagillin-induced chromosomal aberrations at 10−20 mg/kg in mouse bone marrow cells[46]. In summary, the Stanimirovic? group concluded that fumagillin DCH is a mutagenic formulation, both in vitro and in vivo.
After the treatment of Nosema apis-infected bees with fumagillin the electron-density of the mitochondrial matrix in corpora allata increased and the dimensions of the mitochondria diminished in comparison with untreated infected bees.[47]. Fumagillin produced certain effects on secretion granules of hypopharyngeal glands of bees which increased and had homogeneous structure, which is explained by the changes in secretory activities of gland[48]. It was confirmed that fumagillin largely increased mortality in bees as well as the number of fungi.
Common side effects in human trials in which fumagillin is administered orally are gastrointestinal-related cramping, diarrhea, and significant loss of body weight[40]. This undesirable weight loss side effect prompted recent, perhaps ethically questionable, trial use of fumagillin as a chemical mitigation for obesity[41].
Fumagillin was observed to exhibit significant negative chromosomal aberration effects at 50−75 mg/kg body weight in mice. Concentrations of 25, 50, and 75 mg/kg body weight were administered by gavage[42]. All experimental dosages listed above induced significant antiproliferative and genotoxic potential in mice[43]. Fumagillin exhibited clastogenic activity in human lymphocytes at concentrations equivalent to the therapeutic dose in beekeeping[44]. Genotoxicity to mouse bone marrow cells at concentrations of 10−20 mg/kg body weight administered in vivo to mice by gastric probe (5, 10, and 20 mg/kg concentrations tested), as compared to a control group, was also observed[45]. These results were confirmed with fumagillin-induced chromosomal aberrations at 10−20 mg/kg in mouse bone marrow cells[46]. In summary, the Stanimirovic? group concluded that fumagillin DCH is a mutagenic formulation, both in vitro and in vivo.
After the treatment of Nosema apis-infected bees with fumagillin the electron-density of the mitochondrial matrix in corpora allata increased and the dimensions of the mitochondria diminished in comparison with untreated infected bees.[47]. Fumagillin produced certain effects on secretion granules of hypopharyngeal glands of bees which increased and had homogeneous structure, which is explained by the changes in secretory activities of gland[48]. It was confirmed that fumagillin largely increased mortality in bees as well as the number of fungi.
- MOLINA, J.M., L. GOGUEL, C. SARFATI, J.F. MICHIELS, I. DESPORTES-LIVAGEL, S. BALKAN et al.[2000]: Trial of oral fumagillin for the treatment of intestinal microsporidiosis in patients with HIV infection. AIDS 14, 1341-1348.
- CONTEAS, C.N., O.G. BERLIN, L.R. ASH, J.S. PRUTHI[2000]: Therapy for human gastrointestinal microsporidiosis. Am J Trop Med Hyg. 63, 121-127.
- BAILEY, L.[1953]: Effect of fumagillin upon Nosema apis[Zander]. Nature 171:112-213.
- EL-MATBOULI, M., R.W. HOFFMANN[1991]: Prevention of experimentally induced whirling disease in rainbow trout Oncorhynchus mykss by Fumagillin. Dis Aquat Organ. 10, 109-113.
- MORRIS, D.J., A. ADAMS, P. SMITH, R.H. RICHARDS[2003]: Effects of oral treatment with TNP-470 on rainbow trout[Oncorhynchus myksis] infected with T etracapsuloides bryosalmonae[Malacosporea], the causative agent of proliferative kidney disease. Aquaculture 221, 51-64.
- FURGALA, B.[1962]: Residual fumagillin activity in sugar syrup stored by wintering honeybee colonies. J Apic Res. 1, 35-37.
- ASSIL, H.I., P. SPORNS[1991]: ELISA and HPLC methods for analysis of fumagillin and its decomposition products in honey. J Agr Food Chem. 39, 2206-2213.
- KATZNELSON, H., C.A. JAMIESON[1952]: Control of Nosema disease of honey-bees with fumagillin. Science 115, 70-71.
- HEDRICK, R.P., J.M. GROFF, T. MCDOWELL[1988]: Oral administration of Fumagillin DCH protects Chinook salmon Oncorhynchus tshawytscha from experimentally-induced proliferative kidney disease. Dis Aquat Organ. 4, 165-168.
- KENT, M.L., S.C. DAWE[1994]: Efficacy of fumagillin DCH against experimentally-induced Loma salmonee[Microsporea] infections in Chinook salmon Oncorhynchus tshawytscha. Dis Aquat Organ. 20,231-133.
- LE GOUVELLO, R., T. POBEL, R.H. RICHARDS, C. GOULD[1999]: Field efficacy of a 10-day treatment of fumagillin against proliferative kidney disease in rainbow trout Oncorhynchus mykiss. Aquaculture 171, 27-20.
- ROSERGER, D.F., O.N. SERDARA VIC, R.A. EVLANDSON, R.T. BRY AN, D.A. SCHW ARTZ et al.[1993]: Successful tretmant of mikrosporidial keratoconjuctivitis with topical fumagillin in a patient with AIDS. Cornea 12, 261-265.
- WILKINS, J.H., N. JOSHI, T.P. MARGOLIS, V. CEVALLOS, D.R. DAWSON[1994]: Microsporidial keratoconjunctivitis treated successfully with a short coures of fumagillin. Eye 8: 703-704.
- MOLINA, J.M., M. TOURNEUR, C. SARFATI, S. CHEVRET, A. DE GOUVELLO et al.[2002]: Fumagillin treatment of intestinal microsporidiosis. New Engl J Med. 346, 1963-1969.
- CONTEAS, C.N., O.G. BERLIN, L.R. ASH, J.S. PRUTHI[2000]: Therapy for human gastrointestinal microsporidiosis. Am J Trop Med Hyg. 63, 121-127.
- Genersch, E. Honey bee pathology: current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87−97.
- Didier, E. S.; Weiss, L. M. Overview of microsporidia and microsporidiosis. Protistology 2008, 5, 243−255.
- Franzen, C. Microsporidia: a review of 150 years of research. Open Parasitol. J. 2008, 2, 1−34.
- Weiss, L. M.; Edlind, T. D.; Vossbrinck, C. R.; Hashimoto, T. Microsporidian molecular phylogeny: the fungal connection. J. Eukaryot. Microbiol. 1999, 46, 17S−18S.
- Sina, M.; Alastair, G.; Farmer, M.; Andersen, R.; Anderson, O.; Barta, J.; Bowser, S.; Brugerolle, G.; Fensome, R.; Fredericq, S.; James, T.; Karpov, S.; Kugrens, P.; Krug, J.; Lane, C.; Lewis, L.; Lodge, J.; Lynn, D.; Mann, D.; Maccourt, R.; Mendoza, L.; Moestrup, O.; Mozley, S.; Nerad, T.; Shearer, C.; Smirnov, A.; Spiegel, F.; Taylor, M. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005, 52, 399−451.
- Traver, B. E.; Fell, R. D. Low natural levels of Nosema ceranae in Apis mellifera queens. J. Invertebr. Pathol. 2012, 110, 408−410.
- Higes,M.;Marti?n-Hernan?dez,R.;Garcia-Palencia,P.;Martin, P.; Meana, A. Horizontal transmission of Nosema ceranae[Microsporidia] from worker honeybees to queens[Apis mellifera]. Environ. Microbiol. Rep. 2009, 1, 495−498.
- Smith, M. L. The honey bee parasite Nosema ceranae: transmissible via food exchange? PLoS One 2012, 7, e43319[accessed Feb 25, 2014].
- Goblirsch, M.; Huang, Z. Y.; Spivak, M. Physiological and behavioral changes in honey bees[Apis mellifera] induced by Nosema ceranae infection. PLoS One 2013, 8, e58165[accessed Feb 25, 2014] DOI: 10.1371/journal.pone.0058165.
- Bailey, L.; Ball, B. V. Honey Bee Pathology, 2nd ed.; Academic Press: London, UK, 1991.
- Eble, T. E.; Hanson, F. R. Fumagillin, an antibiotic from Aspergillus fumigatus H-3. Antibiot. Chemother. 1951, 1, 54−58.
- McCowan, M. C.; Callender, M. E.; Lawlis, J. F. Fumagillin[H3], a new antibiotic with amebicidal properties. Science 1951, 113, 202−203.
Chemical Properties
White powder
Uses
Methionine aminopeptidase 2 inhibitor
Uses
Fumagillin is a compound isolated from the fungus Aspergillus fumigatus. Fumagillin is an antimicrobial agent used in the treatment of microsporidiosis. Fumagillin shows promise as both an an anti-inf ective and antiangiogenic agent.
Uses
Fumagillin is a polyene mycotoxin isolated from Aspergillus fumigatus in 1951 as a potent antiprotozoan for the treatment of amoebiasis. More recently, fumagillin has been shown to inhibit endothelial cell proliferation and angiogenesis by inhibiting methionine aminopeptidase-2 (MetAP-2).
Definition
An antibiotic substance produced by Aspergillus fumigatus.
Biological Activity
Antibiotic and antiangiogenic agent; covalently binds and inhibits methionine aminopeptidase-2. Inhibits endothelial cell proliferation in vitro and tumor-induced angiogenesis in vivo . Also inhibits tumor growth in mice. Analog available, TNP 470 (N-(2-Chloroacetyl)carbamic acid (3R,4S,5S,6R)-5-Methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl -2-buten-1-yl)-2-oxiranyl]-1-oxaspiro[2.5]oct-6-yl ester).
Purification Methods
Forty grams of a commercial sample containing 42% fumagillin, 45% sucrose, 10% antifoam agent and 3% of other impurities are digested with 150mL of CHCl3. The insoluble sucrose is filtered off and washed with CHCl3. The combined CHCl3 extracts are evaporated almost to dryness at room temperature under reduced pressure. The residue is triturated with 20mL of MeOH, and the fumagillin is filtered off by suction. It is crystallised twice from 500mL of hot MeOH by standing overnight in a refrigerator (yellow needles). (The long-chain fatty ester used as antifoam agent is still present, but is then removed by repeated digestion, on a steam bath, with 100mL of diethyl ether.) For further purification, the fumagillin (10g) is dissolved in 150mL of 0.2M ammonia, and the insoluble residue is filtered off. The ammonia solution (cooled in running cold water) is then brought to pH 4 by careful addition of M HCl with constant shaking in the presence of 150mL of CHCl3. (Fumagillin is acid-labile and must be removed rapidly from the aqueous acid solution.) The CHCl3 extract is washed several times with distilled water, dried (Na2SO4) and evaporated under reduced pressure. The solid residue is washed with 20mL of MeOH. The fumagillin is filtered off by suction, then crystallised from 200mL of hot MeOH. [Tarbell et al. J Am Chem Soc 77 5610 1955.] Alternatively, 10g of fumagillin in 100mL CHCl3 is passed through a silica gel (5g) column to remove tarry material, and the CHCl3 is evaporated to leave an oil which gives fumagillin on crystallisation from amyl acetate. It recrystallises from MeOH (charcoal) or Me2CO/MeOH. The fumagillin is stored in dark bottles in the absence of oxygen and at low temperatures. [Schenk et al. J Am Chem Soc 77 5606 1955, Beilstein 19 III/IV 1012.]
▼
▲
FUMAGILLIN Preparation Products And Raw materials
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
