

Histamine
| Price | USD3.00 |
| Packge | 3KG |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2019-07-06
Product Details
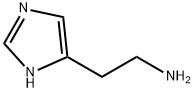
- Product NameHistamine
- CAS No.51-45-6
- EINECS No.200-100-6
- MFC5H9N3
- MW111.15
- InChIKeyNTYJJOPFIAHURM-UHFFFAOYSA-N
- AppearanceSolidWhite to light yellow
- storage temp. -20°C
- Melting point 83-84 °C (lit.)
- Boiling point 167 °C/0.8 mmHg (lit.)
- Water Solubility Soluble in water, alcohol, and hot chloroform.
- density 0.9902 (rough estimate)
| Histamine Basic information |
| Product Name: | Histamine |
| Synonyms: | 4-(2-aminoethyl)-imidazol;4-Imidazoleethylamine;5-Imidazoleethylamine;beta-Aminoethylglyoxaline;beta-Aminoethylimidazole;beta-Imidazolyl-4-ethylamine;Eramin;Ergamine |
| CAS: | 51-45-6 |
| MF: | C5H9N3 |
| MW: | 111.15 |
| EINECS: | 200-100-6 |
| Product Categories: | MI;Bioactive Small Molecules;Building Blocks;C3 to C8;Cell Biology;Chemical Synthesis;H;Heterocyclic Building Blocks;Imidazoles;Inhibitors;Halogenated Heterocycles ,Quinoxalines ,Quinolines ,Quinazolines |
| Mol File: | 51-45-6.mol |
 |
|
| Histamine Chemical Properties |
| Melting point | 83-84 °C(lit.) |
| Boiling point | 167 °C0.8 mm Hg(lit.) |
| density | 0.9902 (rough estimate) |
| refractive index | 1.4690 (estimate) |
| storage temp. | −20°C |
| pka | 6.04(at 25℃) |
| form | Solid |
| color | White to light yellow |
| Water Solubility | Soluble in water, alcohol, and hot chloroform. |
| Merck | 13,4739 |
| BRN | 2012 |
| InChIKey | NTYJJOPFIAHURM-UHFFFAOYSA-N |
| CAS DataBase Reference | 51-45-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Histamine(51-45-6) |
| EPA Substance Registry System | 1H-Imidazole-4-ethanamine(51-45-6) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38-42/43 |
| Safety Statements | 22-26-36/37 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | MS1050000 |
| F | 3-10-23 |
| TSCA | Yes |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29332900 |
| Hazardous Substances Data | 51-45-6(Hazardous Substances Data) |
| Toxicity | LD50 i.p. in mice: 2020 mg/kg (Nagai) |
| MSDS Information |
| Provider | Language |
|---|---|
| 4-(2-Aminoethyl)-1H-imidazole | English |
| SigmaAldrich | English |
| Histamine Usage And Synthesis |
| Chemical Properties | White to slightly yellow powder |
| Uses | H1&2 agonist, edema induction, gastric secretion stimulant |
| Definition | ChEBI: A member of the class of imidazoles that is 1H-imidazole substituted at position C-4 by a 2-aminoethyl group. |
| Indications | Sinus problems, hay fever, bronchial asthma, hives, eczema, contact dermatitis, food allergies, and reactions to drugs are all allergic reactions associated with the release of histamine and other autocoids, such as serotonin, leukotrienes, and prostaglandins. Histamine release is frequently associated with various inflammatory states and may be increased in urticarial reactions, mastocytosis, and basophilia. Histamine also acts as a neurotransmitter in the central nervous system (CNS). Upon release from its storage sites, histamine exerts effects ranging from mild irritation and itching to anaphylactic shock and eventual death. |
| Biosynthesis | Virtually all of the histamine found in individual organs and tissues is synthesized locally and stored in subcellular secretory granules. Within the tissues, the mast cells are the principal sites of storage; in the blood, the basophils serve this function. Histamine is also present in neurons of the CNS, where it acts as a neurotransmitter. Histamine is synthesized from the amino acid histidine by an action of the enzyme histidine decarboxylase. Following synthesis, histamine is either rapidly inactivated or stored in the secretory granules of mast cells and basophils as an inactive complex with proteases and heparin sulfate or chondroitin sulfate. |
| Biological Functions | Histamine occurs in the brain, particularly in certain hypothalamic neurons, and evidence is strong that histamine is a neurotransmitter. Distribution of histamine, its synthetic enzyme (histidine decarboxylase), and methyl histamine (the major brain metabolite) is not uniform. Possible roles for histamine in the regulation of food and water intake, thermoregulation, hormone release, and sleep have been suggested. |
| Mechanism of action | Non–Antigen-Mediated Release of Histamine Histamine may be released from mast cells by mechanisms that do not require prior sensitization of the immune system. Drugs, high-molecular-weight proteins, venoms, and other substances that damage or disrupt cell membranes can induce the release of histamine. Any thermal or mechanical stress of sufficient intensity also will result in histamine release. Cytotoxic compounds, may release histamine as the result of disruption of cell membranes. |
| Pharmacology | Histamine is found in animal tissues and venoms and in many bacteria and plants.Within the human body, the largest histamine concentrations are in the skin, lungs, and gastrointestinal mucosa, while concentrations are smaller in almost all other organs and tissues.Histamine is present in human plasma at relatively low concentrations (usually less than 0.5 ng/mL); in contrast, wholeblood levels can be as high as 30-fold greater. Substantial quantities of histamine are present in urine, with excretion rates varying from 10 to 40μg per 24 hours. |
| Clinical Use | Histamine has only minor uses in clinical medicine. In the past it was used to diagnose pernicious anemia, in which histamine fails to evoke the usual secretion of gastric acid. Histamine has been used to assess bronchial hyperreactivity, although this test may be quite hazardous for asthmatics. Today the main clinical use of histamine is as a positive control injection for allergy skin testing. |
| Side effects | Sedation is the most frequent adverse reaction to the first-generation antihistamines. An additive effect on alertness and motor skills will result if alcohol or another depressant is taken with these drugs. Antimuscarinic effects caused by these drugs include dry mouth and respiratory passages, urinary retention, and dysuria. Nausea, vomiting, constipation or diarrhea, dizziness, insomnia, nervousness, and fatigue also have been reported. Drug allergy, especially after topical application, is fairly common.Tolerance to certain antihistamines may develop after prolonged administration. Teratogenic effects of the piperazine antihistamines have been shown in animal studies. Epidemiological studies have not shown such an association in humans. The effects of toxic doses of first-generation antihistamines, similar to those seen following atropine administration, include excitement, hallucinations, dry mouth, dilated pupils, flushing, convulsions, urinary retention, sinus tachycardia, coma, and death. The second-generation H1-antagonists are often referred to as nonsedating antihistamines; however, doses above the usual therapeutic level can cause sleepiness in certain individuals.A more serious adverse effect of some earlier second-generation antihistamines is cardiotoxicity. |
| Purification Methods | It crystallises from *benzene or chloroform. [Beilstein 25 I 628, 25 II 302, 25 III/IV 2049.] |
| Histamine Preparation Products And Raw materials |
| Raw materials | Sulfuric acid -->Benzoyl chloride-->L-Histidine |
| Preparation Products | L-Histidine-->DL-Histidine |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
