

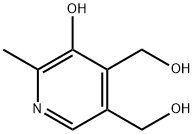
Pyridoxine
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:1kg,5kg,100kg
- Time:2019-07-06
Product Details
- Product NamePyridoxine
- CAS No.65-23-6
- EINECS No.200-603-0
- MFC8H11NO3
- MW169.18
- AppearanceSolidWhite to Off-White
- storage temp. Inert atmosphere,2-8°C
- Melting point 214-215 °C(lit.)
- Boiling point 298.46°C (rough estimate)
- density 1.2435 (rough estimate)
| Pyridoxine Basic information |
| Product Name: | Pyridoxine |
| Synonyms: | 5-hydroxy-6-methyl-4-pyridinedimethanol;Adermin;Beesix;Bezatin;Gravidox;Hexabion;Hydoxin;Pirivitol |
| CAS: | 65-23-6 |
| MF: | C8H11NO3 |
| MW: | 169.18 |
| EINECS: | 200-603-0 |
| Product Categories: | Heterocycle-Pyridine series;GANTRISIN;-;Inhibitors;vitamin series |
| Mol File: | 65-23-6.mol |
 |
|
| Pyridoxine Chemical Properties |
| Melting point | 214-215 °C(lit.) |
| Boiling point | 298.46°C (rough estimate) |
| density | 1.2435 (rough estimate) |
| refractive index | 1.5100 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 0.1 g/mL at 20 °C, clear, colorless |
| pka | pKa 5.00(H2O t = 25.0 I = 0.15 (mixed)) (Uncertain) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | LXNHXLLTXMVWPM-UHFFFAOYSA-N |
| CAS DataBase Reference | 65-23-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Methyl-3-hydroxy-4,5-dihydroxymethylpyridine(65-23-6) |
| EPA Substance Registry System | 3,4-Pyridinedimethanol, 5-hydroxy-6-methyl-(65-23-6) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| RTECS | UV1350000 |
| F | 8-10 |
| HS Code | 29362500 |
| MSDS Information |
| Provider | Language |
|---|---|
| 2-Methyl-3-hydroxy-4,5-dihydroxymethylpyridine | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Pyridoxine Usage And Synthesis |
| Description | Pyridoxine hydrochloride provides pyridoxine, which is also known as vitamin B6. Vitamin B6 is naturally found in foods such as cereals, beans, vegetables, liver, meat, and eggs. Vitamin B6 functionalizes as a coenzyme in the metabolism of protein, carbohydrate, and fat. Vitamin 6 is needed to maintain the health of nerves, skin, and red blood cells. Pyridoxine is used to prevent or treat vitamin B6 deficiency caused by inadequate dietary intake. It is also used to treat drug induced deficiency in patients taking isoniazid or oral contraceptives. |
| References | [1] http://www.webmd.com [2] https://dailymed.nlm.nih.gov |
| Chemical Properties | crystalline solid |
| Uses | antibacterial |
| Uses | A form of vitamin B6 |
| Uses | pyridoxine HCL is a skin-conditioning agent that is also widely used in hair products. |
| Uses | Vitamin B6, a water-soluble vitamin with a solubility of 1 g in 5 ml of water. It functions in the utilization of protein and is an essential nutrient in enzyme reactions. It is necessary for proper growth. During processing, there is a loss due to leaching of the vitamin in water. It is destroyed by high temperatures, high irradiation, and exposure to light. During storage, loss increases with temperature and storage time. It is found in liver, eggs, and meats. |
| Definition | ChEBI: A hydroxymethylpyridine with hydroxymethyl groups at positions 4 and 5, a hydroxy group at position 3 and a methyl group at position 2. The 4-methanol form of vitamin B6, it is converted intoto pyridoxal phosphate which is a coenzyme f r synthesis of amino acids, neurotransmitters, sphingolipids and aminolevulinic acid. |
| Brand name | Hexa-Betalin (Lilly). |
| World Health Organization (WHO) | Pyridoxine (vitamin B6) is listed in theWHO Model List of Essential Drugs. |
| General Description | The discovery of vitamin B6 is generally ascribed to Paul Gy?rgy who first realized there was a vitamin that was distinctly different from vitamin B2 in 1934. Pyridoxine (PN) is the C4 hydroxymethyl derivative, pyridoxal (PL) is the C4 formyl derivative and pyridoxamine (PM) is the C4 aminomethyl derivative of 5-(hydroxymethyl)- 2-methylpyridin-3-ol). Each of these are also converted to their corresponding 5'-phosphate derivatives referred to as pyridoxine 5'-phosphate (PNP), pyridoxal 5'-phosphate (PLP), and pyridoxamine 5'-phosphate (PMP), respectively . Because of their ability to interconvert, all are considered active forms of vitamin B6 in vivo. Although PLP is the major coenzyme form, PMP can also function as a coenzyme primarily in aminotransferases. The major metabolite is 4-pyridoxic acid, which is excreted in the urine. |
| Clinical Use | Pyridoxine is indicated in the treatment and prevention of known or suspected vitamin B6deficiency, which is most likely to occur in the setting of alcoholism in developed countries. At least seven genetic disorders that result in a vitamin B6 deficiency syndrome in the presence of an adequate dietary intake have been identified. These result from defects in enzymes that are responsible for the bioactivation or utilization of vitamin B6. |
| Safety Profile | Moderately toxic by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects: ataxia, local anesthetic, paresthesia. When heated to decomposition it emits toxic fumes of Nox |
| Enzyme inhibitor | This nutritionally essential factor (FWfree base = 169.18 g/mol; CAS 65-23-6), also called vitamin B6 and pyridoxol, is a white solid that is typically supplied as the hydrochloride (the two pKa values are 4.94 and 8.89). (Note: The term vitamin B6 actually refers to a mixture of pyridoxine, pyridoxal, and pyridoxamine.) Pyridoxine is stable in light and air, but is somewhat photolabile in neutral and alkaline solutions. It is soluble in water (1 g/4.5 mL) and has lmax values at 253 and 325 nm at pH 7 in phosphate buffer (e = 3700 and 7100 M–1cm–1, respectively). Pyridoxine is a substrate of the reactions catalyzed by pyridoxine 4-dehydrogenase, pyridoxine 4-oxidase, pyridoxine 5-dehydrogenase, and pyridoxine 5'-O-b-D-glucosyltransferase. Target(s): alanine racemase; arginine decarboxylase; dextransucrase; b-fructofuranosidase, or invertase; glycogen phosphorylase b; hydroxymethylpyrimidine kinase, also alternative substrate; Ki = 2.7 μM; pyridoxal dehydrogenase; pyridoxamine-5-phosphate oxidase; pyridoxamine:pyruvate aminotransferase; and tyrosinase. |
| Pyridoxine Preparation Products And Raw materials |
| Preparation Products | Pyridoxal phosphate-->5-CHLOROMETHYL-3-HYDROXY-4-HYDROXYMETHYL-2-METHYLPYRIDINE |
| Raw materials | D(+)-Sucrose |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Humble Healthcare Limited
- 65-23-6 99%
- Inquiry
- 2024-01-30
-
VIP1年
- Merck Ltd
- 65-23-6 Pyridoxine 98%
- Inquiry
- 2024-01-17
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
