

Oxalic acid dihydrate
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:1kg,5kg,100kg
- Time:2019-07-06
Product Details
- Product NameOxalic acid dihydrate
- CAS No.6153-56-6
- EINECS No.612-167-2
- MFC2H6O6
- MW126.07
- InChIKeyGEVPUGOOGXGPIO-UHFFFAOYSA-N
- AppearancePowder/SolidYellow to yellow-green
- Water Solubility 138 g/L (20 ºC)
- storage temp. Store at +5°C to +30°C.
- Boiling point 108-109°C
- density 1,65 g/cm3
- Melting point 104-106 °C(lit.)
| Product Name: | Oxalic acid dihydrate |
| Synonyms: | OXALIC ACID-2-HYDRATE TECHNICAL;OXALIC ACID-2-HYDRATE EXTRA PURE;OXALIC ACID 2H2O;OXALIC ACID-2-HYDRATE;OXALIC ACID DIHYDRATE;Ethandionic acid, dihydrate;DIMETHYL OXYLATE DIHYDRATE;OxalicAcidGr |
| CAS: | 6153-56-6 |
| MF: | C2H6O6 |
| MW: | 126.07 |
| EINECS: | 612-167-2 |
| Product Categories: | Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;others;C1 to C5;Essential Chemicals;Inorganic Salts;Lipid Library;Metabolic Pathways;Metabolites and Cofactors on the Metabolic Pathways Chart;Reagent Plus;Research Essentials;Solutions and Reagents;Building Blocks;Carbonyl Compounds;Carboxylic Acids;-;Chemical Synthesis;Lipid;Metabolic Libraries;Metabolomics;Organic Building Blocks;ACS Grade |
| Mol File: | 6153-56-6.mol |
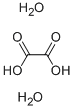 |
|
| Oxalic acid dihydrate Chemical Properties |
| Melting point | 104-106 °C(lit.) |
| Boiling point | 108-109°C |
| density | 1,65 g/cm3 |
| vapor density | 4.4 (vs air) |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| Fp | 157°C |
| storage temp. | Store at RT. |
| solubility | H2O: soluble1M at 20°C, clear, colorless |
| form | Powder/Solid |
| Specific Gravity | 1.65 |
| color | Yellow to yellow-green |
| PH | ~1.0 (25℃, 1M in H2O) |
| Water Solubility | 138 g/L (20 ºC) |
| Sublimation | 157 ºC |
| Merck | 14,6911 |
| BRN | 3679436 |
| Exposure limits | TLV-TWA for anhydrous acid 1 mg/m3 (ACGIH, MSHA, and OSHA); TLV-STEL 2 mg/m3(ACGIH). |
| Stability: | Stable. Incompatible with bases, acid chlorides, steel, silver, silver compounds, moisture. Avoid contact with metals. |
| InChIKey | GEVPUGOOGXGPIO-UHFFFAOYSA-N |
| CAS DataBase Reference | 6153-56-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Oxalic acid dihydrate(6153-56-6) |
| EPA Substance Registry System | Ethanedioic acid, dihydrate(6153-56-6) |
| Safety Information |
| Hazard Codes | Xn,C |
| Risk Statements | 21/22-41 |
| Safety Statements | 24/25-39-37-36-26 |
| RIDADR | UN 3261 8/PG 3 |
| WGK Germany | 1 |
| RTECS | RO2450000 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 29171100 |
| Toxicity | LD50 orally in Rabbit: 375 mg/kg |
| MSDS Information |
| Provider | Language |
|---|---|
| Ethanedionic acid | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Oxalic acid dihydrate Usage And Synthesis |
| Chemical Properties | white crystals |
| Uses | Oxalic acid dihydrate is a purifying agent in pharmaceutical industry, special in antibiotic medication, such as Oxytetracycline , Chloramphenicol , etc; * Precipitating agent in Rare-earth mineral processing; * Bleaching agent in the textile activities, wood pulp bleaching; * Rust-remover for Metal treatment; * Grinding agent, such as Marble polishing; * Waste water treatment, removing calcium from water. |
| Uses | A diprotic reducing agent used as a buffer. |
| Uses | Oxalic acid occurs in the cell sap of Oxalisand Rumex species of plants as the potassium and calcium salt. It is the metabolicproduct of many molds (Merck 1989). Thereare a large number of applications of thiscompound, including indigo dyeing; calicoprinting; removal of paint, rust, and inkstains; metal polishing; bleaching leather; inpesticide compositions and manufacture ofoxalates. It is also used as an analyticalreagent and as a reducing agent in organicsynthesis. Addition of oxalic acid to chromic acid forthe anodizing of Al alloy has been reported tomodify the morphology and improve the corrosion performance of anodic films (Moutarlier et al. 2004). Also, it is a very effectiveadditive for the ozone treatment of cellulose.It prevents the degradation of cellulose fromozone bleaching. |
| Reactivity Profile | At high temperatures oxalic acid decomposes, producing toxic carbon monoxide, andformic acid. Mixing with warm sulfuric acidmay produce the same products: CO2, CO,and formic acid. It reacts with many silvercompounds, forming explosive silver oxalate(NFPA 1986). An explosion occurred whenwater was added to an oxalic acid/sodiumchlorite mixture in a stainless steel beaker.There was also evolution of highly toxicchlorine dioxide gas (MCA 1962). Oxalicacid reacts violently with strong oxidizingsubstances. |
| Health Hazard | Oxalic acid is a strong poison. The toxicsymptoms from ingestion include vomiting, diarrhea, and severe gastrointestinaldisorder, renal damage, shock, convulsions,and coma. Death may result from cardiovascular collapse. The toxicity arises asoxalic acid reacts with calcium in the tissuesto form calcium oxalate, thereby upsettingthe calcium/potassium ratio (ACGIH 1986).Deposition of oxalates in the kidney tubulesmay result in kidney damage (Hodgson et al.1988). Oxalic acid may be absorbed into the bodythrough skin contact. It is corrosive to theskin and eyes, producing burns. Dilute solutions of 10% strength may be a mild irritantto human skin. However, the inhalation toxicity is low because of its low vapor pressure.Airborne dusts can produce eyeburn and irritation of the respiratory tract. LD50 value, oral (rats): 375 mg/kg. |
| Purification Methods | Crystallise oxalic acid from distilled water. Dry it in a vacuum over H2SO4. The anhydrous acid can be obtained by drying at 100o overnight. [Beilstein 2 IV 1819.] |
| Oxalic acid dihydrate Preparation Products And Raw materials |
| Raw materials | Ethylene glycol-->NITRIC OXIDE |
| Preparation Products | Oxalic acid-->N,N,N',N'-Tetramethylethylenediamine-->Vanadyl acetylacetonate-->1-ISOPROPYL-5-HYDROXYPYRAZOL-->2,3-DIHYDROXYQUINOXALINE-6-CARBOXYLIC ACID |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- OXALIC ACID 99%
- Inquiry
- 2024-11-05
-
VIP1年
- Shri Shanti Laboratories
- Oxalic acid dihydrate 6153-56-6 99%
- Inquiry
- 2024-03-28
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
