

Sodium sulfate Manufacturer; In stock GMP Factory
| Price | USD1.00 | USD1.00 |
| Packge | 1KG | 1KG |
- Min. Order:1 KG/CTN
- Supply Ability:TOP 3 largest production factory in China
- Time:2020-06-02
Product Details
- Product NameSodium sulfate Manufacturer; In stock GMP Factory
- CAS No.7757-82-6
- EINECS No.231-820-9
- MFNa2SO4
- MW142.04214
- InChIKeyPMZURENOXWZQFD-UHFFFAOYSA-L
- Appearancepowder (fine)White
- storage temp. Store at +5°C to +30°C.
- Water Solubility 18.5 mg/L
- Melting point 884 °C (lit.)
- density 2.68 g/mL at 25 °C (lit.)
- Boiling point 1700°C
| Product Name: | Sodium sulfate |
| Synonyms: | PHENOL SATURATED II (LOW PH) BIOTECH GR;SODIUM SULPHATE FINE PWDR;Sodium sulfate, anhydrous, 99.99% trace metals basis;Sodium sulfate, 99.995% trace metals basis;Sodium sulfate anhydrous for analysis EMSURE ACS,ISO,Reag. Ph Eur;Sodium sulfate anhydrous granulated for organic trace analysis EMSURE;Sodium sulfate anhydrous, coarse granules for analysis 0.63 - 2.0 mm EMSURE ACS;Sodium sulfate anhydrous, free-flowing, Redi-Dri, ACS reagent, >=99% |
| CAS: | 7757-82-6 |
| MF: | Na2O4S |
| MW: | 142.04214 |
| EINECS: | 231-820-9 |
| Product Categories: | Food Additives;Inorganics;Drying AgentsPesticides&Metabolites;Analytical Reagents;OthersAlphabetic;Pesticides;S;SN - SZ;Special Applications;ACS GradeEssential Chemicals;Salts;Sodium Salts;SodiumMetal and Ceramic Science;Optimization Reagents;Protein Structural Analysis;X-Ray Crystallography;Inorganic Salts;Synthetic Reagents;ACS GradeSample Preparation/Purification;Bulk Treated Silica Gels/Sodium SulfateEssential Chemicals;Other Drying AgentsSynthetic Reagents;Adsorbents, Filter Aids and Drying Agents;Dioxin Sample Prep System;Essential Chemicals;Routine Reagents;Pharmacopoeia (USP);Pharmacopoeia A-ZPharmacopoeia (USP);Pharmacopoeial Inorganics;BioUltraProtein Structural Analysis;DNA&RNA Purification;Molecular Biology;Molecular Biology Reagents;Optimization ReagentsMolecular Biology;Reagents;Anionic SolutionsChromatography;Anionic Standard SolutionsAlphabetic;Application CRMs;Ion Chromatography;Ion Chromatography Standards;S - Z;Salt Solutions;Volumetric Solutions;HPLC;HPLC Buffer;HPLC Buffer - SolidChromatography/CE Reagents;HPLC Buffers;Solid;metal sulfate |
| Mol File: | 7757-82-6.mol |
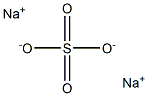 |
|
| Sodium sulfate Chemical Properties |
| Melting point | 884 °C(lit.) |
| Boiling point | 1700°C |
| density | 2.68 g/mL at 25 °C(lit.) |
| refractive index | 1.484 |
| storage temp. | Store at RT. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | powder (fine) |
| color | White |
| PH | 5.2-8.0 (50g/l, H2O, 20℃) |
| Water Solubility | 18.5 mg/L |
| Sensitive | Hygroscopic |
| Merck | 14,8680 |
| Stability: | Stable. Incompatible with strong acids, aluminium, magnesium, strong bases. Hygroscopic. |
| CAS DataBase Reference | 7757-82-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium sulfate(7757-82-6) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 24/25 |
| WGK Germany | 1 |
| RTECS | WE1650000 |
| F | 3 |
| TSCA | Yes |
| HS Code | 28331100 |
| Hazardous Substances Data | 7757-82-6(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| Sodium sulfate | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Sodium sulfate Usage And Synthesis |
| Description | Sodium Sulfate, Na2S04, also known as thenardite and salt cake, is a crystalline compound that melts at 888°C (1632°F). Sodium Sulfate is found in natural form(thenardite) in Chile and Spain. It is used in the manufacture of paperboard, glass,and freezing mixtures. The hydrate, Na2S04·10H20, also known as "Glauber's salt," is a white water-soluble solid formed by heating Sodium chloride and sulfuric acid. It is used in dyeing,manufacturing glass,and in the preparation of sodium bisulfate. |
| Chemical raw materials | Sodium sulfate is an important chemical raw material and is the main raw material for production of sodium sulfide, sodium silicate and other chemical products. It can also be used as filler of synthetic detergent. In the paper industry, it can be used as the cooking agent for the manufacturing of paper pulp of sulfate. Sodium sulfate, also known as anhydrous Glauber's salt or anhydrous mirabilite, appears as white monoclinic crystal or fine powder with the relative density being 2.68 and the melting point being 884 ℃. It can be dissolved in water with the aqueous solution being neutral. It is soluble in glycerol and insoluble in ethanol. It is easy to absorb water when being exposed to become hydrous sodium sulfate. It is a homogeneous polycrystalline material and is rhombus at a temperature of 32.4~223 ℃ with generating shaped monoclinic crystal at higher temperatures and generating hexagonal crystals in 241 ℃. When being crystallized from solution, it has high affinity to iron, iron compound and various kinds of other organic compounds; high-purity, fine-particle anhydrous sodium sulfate is called as “YuanMingFen”. There are two kinds of crystalline hydrate compound of sodium sulfate: one is heptahydrate sodium Na2SO4·7H2O, being white sixty or tetragonal crystal with dehydrating at 24.4 ℃. Another is sodium sulfate decahydrate Na2SO4·10H2O with conventional name being Glauber's salt, “YuanMingFen”, insurance powder. It appears as colorless monoclinic crystal with the density being 1.464g/cm3, the melting point of 32.38 ℃ and loss of crystal water to becomes anhydrous sodium sulfate at 100 ℃. It is easily soluble in water and is easily weathered in dry air to become a dry white powder. Sodium sulfate can be used as chemical analysis reagent such as dye testing; printing industry apply it as mordant agent for making sodium sulfide, glass, water glass, enamel, quantitative measurement of nitrogen and papermaking pulp. In medical care field, it can be used as the antidotes of medical diuretics, laxatives and barium salt poisoning. There are also sodium salt crystals precipitated from salt ponds in winter. Naturally occurring sulfate mineral is widely distributed with mostly existing in magnesium sulfate (calcium) complex salt and mirabilite. There are relative large amount of salt lakes in China's Inner Mongolia, Qinghai, Tibet, and some places of Xinjiang. There is large amount of sodium sulfate in the salt lake. Industrial preparation of sodium sulfate is mainly isolated from natural mineral or by the mirabilite dehydration and treatment with sulfuric acid to obtain sodium chloride. Sodium sulfate is also the byproduct of a lot of chemical industrial production sector and can be extracted from the waste liquid discharged during the production of viscose fiber and cellophane. In the production of sodium dichromate, phenols, boric acid, lithium carbonate, and certain pigments, there is also sodium sulfate as byproduct. Sulfate decahydrate, when being heated to the melting point (32.38 ℃), it can be dissolved its own crystal water while generating anhydrous salt. This temperature is the temperature at which the decahydrate salt, anhydrous salt and saturated solution, the three-phases reaches equilibrium temperature. As long as the three-phase coexists, the temperature is constant. Therefore the system can be used for as constant temperature bath or for calibration of temperature. Another feature of sodium sulfate is being prone to become supersaturated solution. |
| The main function | Sodium sulfate is an important raw material in the manufacturing of glass and paper with the maximum usage amount in the paper and cellulose industry which accounts for around 70% of the total amount. Sodium sulfate is the component of synthetic detergent and is a neutral salt. It can be added to the detergent to reduce the surface tension as well as increases the solubility of the detergent. It is also be used as the dye diluent and the auxiliary agent of the dye printing, as direct dyes, sulfur dyes, vat dyes and other accelerant of dyed cotton fiber, as retarding agent for direct dye silk. In the chemical industry, it can be used as the raw material for the manufacturing of sodium sulfide, gypsum, sodium silicate and other chemical products. It has been now developed of the method for making sulfuric acid, sulfur, soda ash, ammonium sulfate and other products with sodium sulfate. However, due to the high cost of these methods, it has not yet been widely applied. It has been commonly used by laboratory system for using sodium sulfate as cold agent. Glauber's salt has been used as a laxative medicine. The sodium sulfate entering into the body is largely stuck in the gastrointestinal tract without being absorbed and being maintained at certain penetration. It can increase the volume of the intestines and induce intestinal peristalsis with diarrhea effect. Sodium sulfate is the antidote of barium and lead poisoning. Upon lead poisoning, people can apply gastric lavage with 10% Glauber's salt or orally administer 1 to 2% sodium sulfate solution. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Pharmacological effects | Apply oral administration of small amount with its ionic and osmotic pressure effect being able to slightly stimulate the digestive tract mucosa and causes a slight increase of the gastrointestinal secretion and a slight increase in the movement, therefore it has stomachic effect. Orally administer a large amount with a lot of sodium sulfate being dissolved in large amount of water for oral administration. Since the ions are not easily absorbed and can maintain large amount of water in the intestines and can mechanically stimulate the intestinal mucosa and can soften the fecal matter and accelerate the defecation. It is clinically mainly used in the treatment of large intestine constipation, eliminating the intestinal toxins as well as getting rid of parasites. |
| Solubility in water (g / 100ml) | The amount (grams) can be dissolved per 100 ml of water at different temperatures (℃): 4.9g/0 ℃; 9.1g/10 ℃; 19.5g/20 ℃; 40.8g/30 ℃; 48.8g/40 ℃ 45.3g/60 ℃; 43.7g/80 ℃; 42.7g/90 ℃; 42.5g/100 ℃ |
| Identification test | Sodium test of 5% of the sample liquid (IT-28) and sulfate test (IT-29) result is positive. |
| Content Analysis | Accurately weigh 500 mg of sample pre-dried for 4 h at 105 ℃ and dissolve it in 200ml of water; Add 1 mL of hydrochloric acid and heat to boiling. Under constant stirring, add in portions of a small amount (around 10 mL) of hot barium chloride test solution (TS-37), heat on a steam bath for 1h and filter out the precipitate with further washing with water until being chloride-free. After drying, burn and weigh it with the mass of barium sulphate then multiplying by 0.6086, namely is equivalent to sodium sulfate (of Na2SO3) content. |
| Toxicity | ADI does not make restrictive regulations (FAO/WHO, 2001). GRAS (FDA, §186. 1797, 2000). LD50: 5989mg/kg (mice through oral administration). |
| Chemical Properties | It appears as white monoclinic crystal or powder. It can be dissolved in water with the aqueous solution being alkaline. IT can be dissolved in glycerol and is insoluble in ethanol. |
| Uses | It can be mainly used as the filler of synthetic detergent. Paper industry applies it for the cooking agent during the manufacture of sulfate pulp. Glass industry uses it as substitute of soda. Chemical industry applies it as the raw material for the manufacturing of sodium sulfide, sodium silicate and other chemical products. Textile industry applies it for the formulation of Vinylon spinning coagulation bath. The pharmaceutical industry applies it as laxatives. It can also be used in non-ferrous metallurgy and leather. |
| Production method | Vacuum evaporation method: clarify the natural Glauber's salt after dissolving it; put the clarified solution for vacuum evaporation dehydration, thickening, centrifugation and separation and drying to obtain anhydrous sodium sulfate. The reaction equation is: Na2SO4·10H2O → Na2SO4 + 10H2O Calcium mirabilite method: crush the ore of calcium mirabilite, add water ball for grinding, leach, with the leached Glauber's salt being subject to removal of impurities via filtering out. After the clarification of the filtrate, then perform evaporation for dehydration, centrifugation, drying to obtain the anhydrous sodium sulfate. The reaction is: Na2SO4·CaSO4 + 2H2O → Na2SO4 + CaSO4·2H2O Conversion method uses the byproduct, high and low-temperature salt produced from potassium chloride as the raw material for making anhydrous sodium sulfate. Under certain conditions of temperature and ingredients, through three sections of transformation, magnesium sulfate and sodium chloride have been first converted into bloedite and then further converted to anhydrous sodium magnesium sulfate and finally being converted to anhydrous sodium sulfate. The entire conversion process is a continuous countercurrent operation with pulp relying the mechanical driving and elevation, liquid undergoing reverse overflow via potential difference. The conversion of the first segment is controlled at Ph value of 3 to 4 with reaction at a temperature of 55~70 ℃. The generated white sodium magnesium alumina slurry, after entering into a settler for thickening and then sent to the secondary conversion tank; secondary conversion has Ph of 4~5 and temperature of about 100 ℃; third conversion conditions is controlled at Ph5~6 with the temperature being about 55 ℃. Under stirring, the anhydrous loeweite can react with an aqueous solution of sodium chloride to generate anhydrous sodium sulfate crude product with centrifuge, separation, drying to obtain the anhydrous sodium sulfate to obtain. The reaction equation is: 2NaCl + 2MgSO4 + 4H2O → Na2SO4·MgSO4·4H2O + MgC12 Na2SO4·MgSO4·4H20 → Na2SO4·MgSO4 + 4H2O Na2SO4·MgSO4 + 2NaCl → 2Na2SO4 + MgCl2 This method has high cost, low recycling rate with sulfuric acid consumption and the impurities not being prone to be removed. The resulting product is of poor color, but with simple equipment. Rayon byproduct method: the rayon solidification waste undergoes crystallization, dissolution, neutralization, filtration, concentration, cooling, separation and drying process to obtain anhydrous sodium sulfate. The reaction equation is: H2SO4 + 2NaOH → Na2SO4 + 2H2O |
| Chemical Properties | Sodium sulfate, NaS04, also known as thenardite and salt cake, is a crystalline compound that melts at 888°C (1632°C). Sodium sulfate is found in natural form(thenardite) in Chile and Spain. It is used in the manufacture of paperboard, glass,and freezing mixtures. The hydrate, Na2S04·10H20, also known as "Glauber's salt," is a white water-soluble solid formed by heating sodium chloride and sulfuric acid. It is used in dyeing,manufacturing glass,and in the preparation of sodium bisulfate. |
| Uses | Used in kjeldahl nitrogen determination; drying agent . |
| Uses | sodium sulfate is a filler in the manufacturing of synthetic detergents and soaps and a laboratory reagent. It may enhance the irritant action of certain detergents. |
| Safety Profile | Moderately toxic by intravenous route. Mildly toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic effects. Violent reaction with Al. When heated to decomposition it emits toxic fumes of SOx and Na2O. See also SULFATES. |
| Purification Methods | Crystallise sodium sulfate from water at 30o (1.1mL/g) by cooling to 0o. It becomes anhydrous at 32o. |
| Sodium sulfate Basic information |
| Description Chemical raw materials The main function Pharmacological effects Solubility in water (g / 100ml) Identification test Content Analysis Toxicity Chemical Properties Uses Production method |

Chemwill Asia co.,Ltd is one of the leading manufacturer in CHINA.
Our main production base is located in Xuzhou industry park. We produce a wide range of organics including Active pharmaceutical ingredients(APIs), Veterinary, Indole derivatives, Aromatics, Fluorine, Boronic acids, organocatalysts, chiral building blocks, heterocyclic compounds. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.
Our R&D team masters core technology for process-design of target building block as well as Tailor-made fine chemicals. Our manufactory specialised in extreme temperature conditions (-100ºC to +300ºC), hydrogenations reaction under high pressure, suzuki coupling reaction, photochemical reaction, Mitsunobu reactions, enantioselective reactions and asymmetric synthesis.
Think Chemistry,Think CHemWill

Factory Plant Address:
High-Tech Industrial park,Chemical Economical Development Zone,Xuzhou, Jiangsu-Province, P.R.China
Shanghai office: (Export Department)
Address: 16FL., INDUSTRY & COMMERCIAL BUILDING, NO.45 YAN’AN ROAD(E), 200002, SHANGHAI, CHINA
---any inquiries will be replied within 12 hours.
---golden manufactory under iso/gmp with large stock.
---dedication to quality,we have strict quality control system.
---oem/odm available.
---sample is available for your evaluation & formulation development.
PLS contact
Ms Ella Lee - Deputy Sales Manager
Company Profile Introduction
- Intermediate using for Pharmaceuticals
- Fluorine, boronic acids, unnatural amino acids, cross linkers,chiral building blocks
Think Chemistry,Think CHemWill
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- SODIUM SULPHATE ANHYDROUS 7757-82-6 99%
- Inquiry
- 2024-11-05
-
VIP1年
- SS Reagents and Chemicals
- Sodium Sulphate 7757-82-6 99%
- Inquiry
- 2024-10-24
-
VIP1年
- AKASH PHARMA EXPORTS
- Sodium Sulphate 99%
- Inquiry
- 2024-05-16
-
VIP1年
- Shri Shanti Laboratories
- 7757-82-6 Sodium sulfate 99%
- Inquiry
- 2024-03-28
-
VIP1年
- J P Chemicals And Pharma
- 7757-82-6 98%
- Inquiry
- 2024-03-28
-
VIP1年
- Vishnu Chemicals Ltd
- 7757-82-6 Sodium sulfate 99%
- Inquiry
- 2024-03-18
-
VIP1年
- Shalibhadra Group Of Industries
- Sodium sulfate 98%
- Inquiry
- 2024-03-13
-
VIP1年
- Kezi Industries (Sam Industries)
- 7757-82-6 Sodium sulfate 98%
- Inquiry
- 2024-03-02
-
VIP1年
- Gujarat Alkalies and Chemicals Ltd
- Sodium sulfate 7757-82-6 98%
- Inquiry
- 2024-02-21
-
VIP1年
- Vishal Chemical Industries
- Sodium sulfate 99%
- Inquiry
- 2024-02-17
- Since:2004-09-01
- Address: BLDG 9,No.235 Sanlin road,Pudong new district,Shanghai,China
