


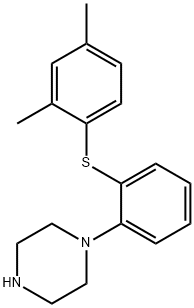
Vortioxetine
| Price | USD2.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100kg
- Time:2019-07-06
Product Details
- Product NameVortioxetine
- CAS No.508233-74-7
- EINECS No.823-919-6
- MFC18H22N2S
- MW298.45
- AppearanceSolidWhite to Off-White
- density 1.16
- Boiling point 424.8±45.0 °C(Predicted)
- storage temp. -20°C Freezer
- Melting point 115-117°C
| Vortioxetine Basic information |
| Product Name: | Vortioxetine |
| Synonyms: | Trintellix (vortioxetine);Vortioxetine(Lu AA21004);1-(2-((2,4-DIMETHYLPHENYL)THIO)PHENYL)PIPERAZINE-HCL;Vortioxetin;Vortioxetine, >=99%;1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine;Lu AA 21004;Vortioxetine |
| CAS: | 508233-74-7 |
| MF: | C18H22N2S |
| MW: | 298.44568 |
| EINECS: | 1308068-626-2 |
| Product Categories: | Inhibitors;5-HT antagonist;Serotonin transporter inhibitor |
| Mol File: | 508233-74-7.mol |
 |
|
| Vortioxetine Chemical Properties |
| density | 1.16 |
| Safety Information |
| Vortioxetine Usage And Synthesis |
| Curative for Major Depression Disorder | Vortioxetine is a new drug for treating depression, jointly developed and researched by the Danish Lundbeck (Lundbeck) and Japanese Takeda. In September 30, 2013, It is approved by the U.S. Food and Drug Administration (FDA) to enter into market with the brand name of Brintellix, for Major Depression Disorder (MDD) treatment. In October 2013, the European Medicines Agency (EMA)’s subordinate agency: Committee for Medicinal Products for Human Use (CHMP) recommended that vortioxetine for the treatment of severe depression should be licensed in the European market and be launched into the European market in January 2014. MDD features mood changes and a series of other symptoms, which have a great impact on the patient's ability to work, sleep, study, eat and enjoy the current happiness. Depressive symptoms can recur many times in life, but some patients may experience only once. Vortioxetine, mainly by increasing the concentration of serotonin (5-HT) in the central nervous system (CNS), exerts antidepressant effects. Compared with other selective serotonin reuptake inhibitors (SSRIS) or serotonin-norepinephrine reuptake inhibitors (SNRIS), vortioxetine almost has no effect on norepinephrine and dopamine neurons. A number of clinical trials have demonstrated the efficacy, safety and tolerability of vortioxetine in the treatment of MDD. 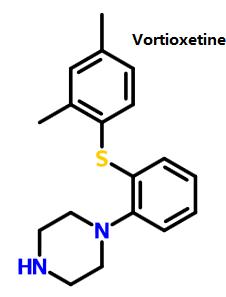 Figure 1 shows the structure of vortioxetine. Figure 1 shows the structure of vortioxetine. |
| Mechanism of Action | Vortioxetine is a small molecule piperazine sulfide. WHO w classifies it as antidepressants (N06A). The drug classification system of European Pharmaceutical Market Research Association (EPhMRA) classifies it as hypnotic / sedative drugs (N5B), antidepressants and mood stabilizers (N6A). Currently, depression is thought to be associated with a decrease in 5-HT activity, and decreased activity of norepinephrine (NE) and dopamine (DA) is thought to be associated with depression. Vortioxetine is a potent serotonin reuptake inhibitor. In human body it has high affinity with serotonin trans-porter (Ki=1.6 nmol - L - 1). But it almost has no affinity with norepinephrine transporter (Ki=113nmol - L-1) and dopamine transporter (Ki= 1000nmol - L - 1). At the same time, it is also a 5-HT1A receptor agonist and 5-HT1B receptor partial agonist, 5-HT1D, 5-HT3 and 5-HT7 receptor antagonist and 5-HT uptake inhibitor. Animal studies have shown that in rats, vinpocetine through interaction on these receptors, will increase depression related brain regions -- the level of the ventral hippocampus, prefrontal cortex extracellular serotonin , dopamine, norepinephrine, acetylcholine and histamine, at the same time it is also regulating the function of y -GABA (γ-aminobutyric acid) and glutamatergic neuron, thus exerting the effect of antidepressant. |
| Pharmacokinetics | The relative molecular weight of vortioxetine is small, and the plasma protein binding rate is 98 percent, which is not related to plasma concentration. It is widely distributed outside the cell and the apparent distribution volume is about 2600 L. Daily oral administration is 2.5 ~ 60mg, showing the linear pharmacokinetic characteristics in proportion to dosage of administration. Bioavailability is 75 percent and generally after 2 weeks of taking vortioxetine, its concentration will be stable in blood. Cmax is 7 to 11 hours. Vortioxetine is metabolized mainly by oxidation and glucuronidation . After a single oral administration of the radioisotope labeled vortioxetine, about 59 percent of it is excreted through the urine, and about 26 percent were excreted through the feces. There was almost no original vortioxetine 48 hours after administration of the drug. The complete metabolism in body requires about 66 hours. The oxidation is mainly completed through cytochrome P450 enzyme (CYP). The involved CYP includes CYP3A4/5, CYP2C19, CYP2C9, CYP2A6, CYP2C8, CYP2B6 and CYP2D6. Among them, CYP2D6 is the key enzyme which catalyzes and produces the main metabolite of duloxetine carboxylic acid. The drug concentration of CYP2D6 in the plasma with slow metabolism is twice higher than that of CYP2D6 in the plasma with the fast metabolism. |
| Drug Interaction |
|
| Synthesis |
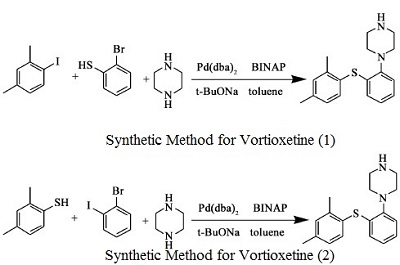 |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Aktinos Pharma Pvt Ltd
- 508233-74-7 Vortioxetine 98%
- Inquiry
- 2024-03-20
-
VIP1年
- Aspen Biopharma Labs Pvt Ltd
- Vortioxetine 508233-74-7 98%
- Inquiry
- 2024-03-16
-
VIP1年
- Exemed Pharmaceuticals (Oneiro Chemicals Pvt Ltd)
- 508233-74-7 Vortioxetine 98%
- Inquiry
- 2024-02-28
-
VIP1年
- Macleods Pharmaceuticals Limited
- Vortioxetine 508233-74-7 98%
- Inquiry
- 2024-01-08
-
VIP1年
- Lupin Ltd
- Vortioxetine 99%
- Inquiry
- 2024-01-05
-
VIP1年
- Varanous Labs Pvt Ltd
- 508233-74-7 98%
- Inquiry
- 2023-12-26
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
