

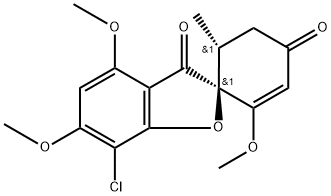
(+)-Griseofulvin
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1g
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product Name (+)-Griseofulvin
- CAS No.126-07-8
- EINECS No.204-767-4
- MFC17H17ClO6
- MW352.77
- InChIKeyDDUHZTYCFQRHIY-ONINOZFXNA-N
- AppearancePowderWhite to yellow-white
- storage temp. 2-8°C
- Water Solubility practically insoluble
- Melting point 218-220 °C(lit.)
- Boiling point 469.04°C (rough estimate)
- density 1.2579 (rough estimate)
| (+)-Griseofulvin Basic information |
| Product Name: | (+)-Griseofulvin |
| Synonyms: | Griseofulvin USP(micronized);(+)-GRISEOFULVIN;GRISEOFULVIN;[2S-TRANS]-7-CHLORO-2,4,6-TRIMETHOXY-6'-METHYLSPIRO[BENZOFURAN-2(3H), 1'(2)-CYCLOHEXANE]-3,4'-DIONE;(2S)-TRANS-7-CHLORO-2',4,6-TRIMETHOXY-6'-METHYLSPIRO(BENZOFURAN-2[3H],1'-[2]CYCLOHEXENE)-3,4'-DIONE;7-CHLORO-2',4,6-TRIMETHOXY-6'-METHYL-, (1'S-TRANS)-SPIRO(BENZOFURAN-2(3H),1'-(2)CYCLOHEXENE)-3,4'-DIONE;(+)-griseofulvi;(2s-trans)-7-chloro-2’,4,6-trimethoxy-6’-methylspiro[benzofuran-2(3h),1’-[2]cy |
| CAS: | 126-07-8 |
| MF: | C17H17ClO6 |
| MW: | 352.77 |
| EINECS: | 204-767-4 |
| Product Categories: | Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Antifungal;Aromatics;Chiral Reagents;VERELAN |
| Mol File: | 126-07-8.mol |
 |
|
| (+)-Griseofulvin Chemical Properties |
| Melting point | 218-220 °C(lit.) |
| alpha | 354 º (c=1, dimethylform) |
| Boiling point | 469.04°C (rough estimate) |
| density | 1.2579 (rough estimate) |
| refractive index | 1.4429 (estimate) |
| storage temp. | −20°C |
| solubility | Practically insoluble in water, freely soluble in dimethylformamide and in tetrachloroethane, slightly soluble in anhydrous ethanol and in methanol |
| form | Powder |
| color | White to yellow-white |
| Water Solubility | practically insoluble |
| λmax | 321nm(CHCl3)(lit.) |
| Merck | 14,4549 |
| BRN | 95226 |
| InChIKey | DDUHZTYCFQRHIY-RBHXEPJQSA-N |
| CAS DataBase Reference | 126-07-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Griseofulvin(126-07-8) |
| EPA Substance Registry System | Spiro[benzofuran- 2(3H),1'-[2]cyclohexene]- 3,4'-dione, 7-chloro-2',4,6-trimethoxy- 6'-methyl-, (1'S,6'R)-(126-07-8) |
| Safety Information |
| Hazard Codes | T,Xn |
| Risk Statements | 60-61-40-43-45 |
| Safety Statements | 53-22-36/37/39-45 |
| WGK Germany | 3 |
| RTECS | WG9800000 |
| Hazardous Substances Data | 126-07-8(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| (+)-Griseofulvin | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| (+)-Griseofulvin Usage And Synthesis |
| General description | Griseofulvin is a non-polyene class antifungal antibiotics; it can strongly inhibit the mitosis of fungal cell and interfere with the fungal DNA synthesis; it can also bind to tubulin to prevent fungal cell division. It has been applied to clinical medicine since 1958 and has currently been widely used for treating the fungal infections of the skin and the stratum corneum with strong inhibitory effects on Trichophyton rubrum and Trichophyton tonsorans, etc. Griseofulvin is not only a widely used antibiotic for clinical treatment of fungal infections of the skin and cuticle, but also applied in agriculture for prevention and treatment of fungal diseases; for example, it has a special efficacy on treating a kind of candidiasis in apple which can cause infection during pollination. |
| Physical and Chemical Properties | Griseofulvin pure product is colorless crystal, being neutral, and insoluble in water with a strong systemic property. It is stable both in vitro and in vivo of plants and is also stable to the various environment factors between pH3.0~8.8. It can be used for prevention and treatment of a variety of fungal plant diseases. The main species china applied for production of griseofulvin is Penicillium nigricans and Penicillium urticae. When grown at the medium, Penicillium nigricans is fuzzy with fresh culture being pure gray and aging culture being dark gray with its back being deep orange-red to the back orange brown. The fungi lawn of Penicillium urticae is yellowish green to pure light gray, thick and dense with the back being pale yellow to brownish; their aerial hyphae has a broom-like shape. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Pharmacological effects | In medicine, this product mainly has good antibacterial effect against Trichophyton, Microspores, and Epidermophyton fungi. However, it has no anti-bacterial effect on Candida, Cryptococcus, Histoplasma, Sporothrix, Blastomyces, and Coccidioides and so on. Mechanism of action of is drug is through interfering with the biosynthesis of nucleic acids of fungal and further inhibiting of its growth. In agriculture, this product has good inhibitory effect on the Ascomycetes, Basidiomycetes, Deuteromycetes and some kinds of algae. However, it is not effective in treating oomycetes whose cell wall containing no chitin content. After spraying melons and other crops, it can be transferred into the branch, leaves and fruits through inner absorption, the absorption of water via rrot, as well as transpiration effect, and further preventing the fungal diseases; according to the study of Brandeis et al. (1946), at a concentration as low as even 10 mg/L or 1 mg/L, griseofulvin can already can make the mycelium of Bctrytis allill be stout, deformed and exhibit spiral bending, also cause: large number of abnormal branching; the development retardation of germinated spores, and loss of apical dominance; thus it has uptake therapeutic effects on a variety of plant diseases, especially powdery mildew, gray mold diseases; Griseofulvin has a more prominent antibacterial activity than the bactericidal activity. |
| Pharmacokinetics | The absorption of orally administration of this drug varies according to the difference in preparation, the micro-particle of this drug can be absorbed by 25% to 70%; its ultrafine particles can almost all absorbed after oral administration. Eating fat can significantly increase the extent of absorption. The product has a serum protein binding rate of being 80%. After its absorption, this product can be deposited in the skin, hair, nail cuticle and combine with keratin to prevent the further invasion of sensitive dermatophytes; at the same time, the pathogenic fungi locate in superficial stratum corneum will leave the body together with the fall-off of the skin or hair with only a very small amount being distribution in other body fluids and tissues. This product can also enter into the fetal circulation and be secreted from the milk. This product is inactivated through metabolism in the liver with the main metabolite being 6-methyl griseofulvin and glucuronic acylate; the blood elimination half-life is 14 to 24 hours. The percentage of product secreted from the urine in its prototype is less than 1% with about 16% to 36% of the prototype being discharged from the feces. |
| Indications | In medicine, this product is suitable for the treatment of a variety of ringworm, including tinea capitis, tinea barbae, body tinea, jock itch, foot tinea and onychomycosis. The various kinds of tinea mentioned are caused by various fungi including Trichophyton rubrum, Trichophyton tonsorans, Trichophyton mentagrophytes, Fingers Trichophyton, etc., and Microsporon audouini, Microsporon canis, Microsporon gypseum and Epidermophyton floccosum, etc. due. This product is not suitable for treatment in mild cases, localized infection cases and cases which can be treated with topical antifungal agents. Griseofulvin is not effective in treating the infections of a various kinds of fungi such as Candida, Histoplasma, Actinomyces, Sporothrix species, Blastomyces, Coccidioides, Nocardio and Cryptococcus species as well as treating tinea versicolor. In agriculture, this product is first introduced by Brian et al (1951) for control of plant diseases. According to previous studies, it can be used for prevention of melon (melon) vine blight, crack spread disease, watermelon blight, anthracnose, apple blossom rot, apple cold rot, apple rot, cucumber downy mildew , strawberry gray mold, gourds hanging blight, powdery mildew of roses, chrysanthemums powdery mildew, rot flower lettuce, early tomato blight, tulip fire blight and other fungal diseases. |
| Side effects | 1. Nervous system headache is relatively common with about 10% of patients experiencing headaches; it is a bit severe at initial stage, but will be alleviated with the continuing administration of drug; other reactions also include lethargy and fatigue. Occasional dizziness, ataxia, and peripheral neuritis may also occur. 2. For Digestive system; a small number of patients may undergo abdominal discomfort, nausea or diarrhea, usually mild which can be tolerated by the patient. 3. Allergies can occur in about 3% of patients which suffers rash; occasionally angioneurotic edema, persistent urticaria, and exfoliative dermatitis can also occur; in very rare cases, photosensitive dermatitis can occur at a small number of patients. 4. This product can sometimes cause reduction of the number of peripheral blood leukocyte; sometime can even cause liver toxicity and proteinuria. |
| Precautions | 1. Cross allergy; because griseofulvin is obtained from Penicillium, which indicated that the drug may have cross-allergy with penicillin or penicillamine, however, this kinds of hypothesis hasn’t been confirmed by clinical cases; but patients allergic to penicillin should still apply with caution and subject to careful observation. 2. Griseofulvin can cause tumor in animal experiments. 3. This product can occasionally cause liver toxicity; patients with original liver disease or liver damage should decide whether to choose to apply this drug after thinking twice. 4. It can induce porphyria and lupus; lupus patients who have index should have trade-off decision before applying this drug. 5. During the treatment, regular testing of peripheral blood, liver function, blood nitrogen content in urea, creatinine and urine routine are demanded. 6. It can be administrated either simultaneously with meal or after the meal with high-fat meal being the best, because they can reduce the gastrointestinal side effects and increase drug absorption. 7. In order to prevent relapse, treatment should be continued until clinical symptoms and laboratory tests confirmed that the pathogen has been completely eradicated. Usually treatment course is: tinea capitis: 8 to 10 weeks; tinea: 2 to 4 weeks; tinea pedis: 4-8 weeks; finger nail tinea: at least four months; nail psoriasis: at least six months, although still with a high recurrence rate. 8. A suitable topical administration is usually necessary at the same time; this is particularly important for athlete's foot. 9. During the treatment or at least 6 months of post-treatment, male patients should take contraceptive measures for prevention of pregnancy. |
| Drug Interactions | 1. Combination of this product together with ethanol can cause tachycardia, sweating, flushing of the skin, etc., so avoid taking them at the same time. 2. Combination with anticoagulant drugs such as warfarin, and coumarin can enhance the hepatic metabolism and leaving the efficacy of anticoagulation being weakened; so it is necessary to adjust the dose by monitoring the prothrombin time at the same time. 3. Combination with primidone, and phenobarbital can decrease the antifungal effect of this product which may be due to that absorption of barbiturates may reduce the absorption of this drug as well as the increased rate of its inactivation due to the induction of hepatic enzyme which causes the decreased blood concentration; thus should avoid this kind of combination of drugs. 4. Combination of estrogen-class contraceptive with the product can reduce the effect of orally administrated contraceptives which may be due to that the product may strengthen the metabolism of contraceptive drugs in the live, causing its decreased blood concentration; thus should avoid using them simultaneously. |
| Toxicity | Griseofulvin has a relatively low toxicity to warm-blooded animals; intraperitoneal injection of both sexes of Waster rats found that they could both withstand a dosage of per 100ml/kg (some damage effects on the seminal vesicles and the intestinal epithelium were observed). Although intravenous injection can cause the temporary inhibition of cells mitosis (especially bone marrow some cells), the rates were almost unaffected under normal conditions. Moreover, after 24 hours, the rats began to rapidly recover; its LD50 is 400ml/kg body weight. Furthermore, even a large dose of griseofulvin only has a very small toxicity to the plants. It will be slowly degraded in the body with a faster degradation rate in soil; so there are no issues about residue and environmental pollution problems. However, the property of its fast degradation also limits their application of antimicrobial activity in agriculture. |
| Pediatric medication | We are lack of information about the medication on children under 2 years. |
| Elderly medication | There are still no existing information about its application in Elderly people and the relation with their ages. |
| Chemical Properties | It is white or white-like powder. Melting point is 218-224 °C. It is highly soluble in tetrachloroethane, soluble in acetone or chloroform, slightly soluble in methanol or ethanol, and very slightly soluble in water. It is odorless or almost odorless with slightly bitter taste. It is stable to heat. |
| Production methods | Griseofulvin is a antibiotic produced from the culture fermentation broth of Penicillium griseofulvum. |
| Chemical Properties | Crystalline Solid |
| Category | Pesticide |
| Uses | adrenegic blocker, Ca channel blocker, coronary vasodilator, antiarrhythmic |
| Uses | antifungal, inhibits mitosis in metaphase |
| Uses | Griseofulvin is a spirobenzofuran produced by a number of Penicillium species, first isolated in the 1930s by Raistrick's group. Griseofulvin is a selective antifungal agent used to treat skin infections in animals and humans. Griseofulvin acts by binding to fungal tubulin and inhibiting the mitotic spindle. Griseofulvin's ability to bind to keratin is considered an important aspect of the metabolite's access to dermatophytic fungi. More recently, griseofulvin has become an important phenotypic marker in Penicillium taxonomy. |
| Uses | An antifungal and antiproliferative agent that affects microtubules. |
| Uses | It is an antifungal drug. It is used both in animal and in humans, to treat rigworm infections of the skin and nails. It is derived from the mold Penicillium griseofulvum |
| Definition | ChEBI: An oxaspiro compound produced by Penicillium griseofulvum. It is used by mouth as an antifungal drug for infections involving the scalp, hair, nails and skin that do not respond to topical treatment. |
| Brand name | Fulvicin Bolus [Veterinary] (Schering-Plough Animal Health); Fulvicin-P/G (Schering); Fulvicin-U/F (Schering); Fulvicin-U/F Powder and Tablets [Veterinary] (Schering-Plough Animal Health); Grifulvin V (Ortho Pharmaceutical); Grisactin (Wyeth- Ayerst); Gris-PEG (Allergan Herbert). |
| Toxicity grading | Low toxicity |
| Acute toxicity | Oral-rat LD50: 10000 mg/kg; Oral-Mouse LD50: 50000 mg/kg |
| Flammability hazard characteristics | Combustion can produce toxic chloride gas |
| Storage Characteristics | Treasury: ventilation low-temperature an dry; it should be separately stored and transported from raw materials of food. |
| Extinguishing agent | Dry powder, foam, sand |
| General Description | White to pale cream-colored crystalline powder. Odorless or almost odorless. Tasteless. Sublimes without decomposition at 410°F. |
| Air & Water Reactions | Insoluble in water. |
| Reactivity Profile | (+)-Griseofulvin is incompatible with strong oxidizing agents. . |
| Hazard | Possible carcinogen. |
| Fire Hazard | Flash point data for (+)-Griseofulvin are not available. (+)-Griseofulvin is probably combustible. |
| Purification Methods | Crystallise it from *benzene or EtOH. Purify 2g of griseofulvin by chromatography on Alumina (40 x 1.5cm) and elute with *C6H6/MeOH (199:1) and follow the UV blue fluorescent band. [MacMillan J Chem Soc 1823 1959, Beilstein 18 III/IV 3160, 18/5 V 150.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Medi Pharma Drug House
- Griseofulvin 99%
- Inquiry
- 2023-12-28
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
