

Isopropyl ether
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1g
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameIsopropyl ether
- CAS No. 108-20-3
- EINECS No.203-560-6
- MFC6H14O
- MW102.17
- InChIKeyZAFNJMIOTHYJRJ-UHFFFAOYSA-N
- AppearanceLiquidAPHA: ≤25
- Water Solubility 9 g/L (20 ºC)
- Melting point -85.5 °C
- Boiling point 68-69 °C(lit.)
- density 0.725 g/mL at 25 °C(lit.)
- storage temp. Store below +30°C.
| Isopropyl ether Basic information |
| Product Name: | Isopropyl ether |
| Synonyms: | (iso-C3H7)2O;1,1'-Dimethyldiethyl ether;1-methyl-1-(1-methylethoxy)ethane;2,2’-oxybis-propan;2,2’-oxybispropane;2,2’-oxybis-Propane;2,2'-Oxybispropane;2,4-dimethyl-3-oxapentane |
| CAS: | 108-20-3 |
| MF: | C6H14O |
| MW: | 102.17 |
| EINECS: | 203-560-6 |
| Product Categories: | ACS and Reagent Grade Solvents;ACS Grade;ACS Grade Solvents;Amber Glass Bottles;Carbon Steel Cans with NPT Threads;Semi-Bulk Solvents;Solvent Bottles;Solvent by Application;Solvent Packaging Options;Solvents;Analytical Reagents;Analytical Reagents for General Use;Analytical/Chromatography;C-D;Puriss p.a.;Puriss p.a. ACS;Aluminum Bottles |
| Mol File: | 108-20-3.mol |
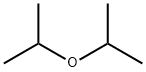 |
|
| Isopropyl ether Chemical Properties |
| Melting point | -85.5 °C |
| Boiling point | 68-69 °C(lit.) |
| density | 0.725 g/mL at 25 °C(lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 120 mm Hg ( 20 °C) |
| refractive index | n20/D 1.367(lit.) |
| Fp | −29 °F |
| storage temp. | Flammables area |
| solubility | 3.11g/l |
| form | Liquid |
| color | APHA: ≤25 |
| explosive limit | 1-21%, 100°F |
| Water Solubility | 9 g/L (20 ºC) |
| Merck | 14,5212 |
| BRN | 1731256 |
| Stability: | Stability Extremely flammable. This material is a serious fire and explosion risk. Vapour may travel considerable distances to an ignition source, which need not be an open flame, but may be a hot plate, steam pipe, etc. Vapour may be ignited by the static electricty which can build up when isopropyl ether is being poured from one vessel |
| CAS DataBase Reference | 108-20-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Diisopropyl ether(108-20-3) |
| EPA Substance Registry System | Propane, 2,2'-oxybis-(108-20-3) |
| Safety Information |
| Hazard Codes | F |
| Risk Statements | 11-19-66-67-52/53 |
| Safety Statements | 9-16-29-33-61 |
| RIDADR | UN 1159 3/PG 2 |
| WGK Germany | 1 |
| RTECS | TZ5425000 |
| F | 10 |
| HazardClass | 3 |
| PackingGroup | II |
| Hazardous Substances Data | 108-20-3(Hazardous Substances Data) |
| Toxicity | LD50 in 14 day old, young adult, adult rats (ml/kg): 6.4, 16.5, 16.0 orally (Kimura) |
| MSDS Information |
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| Isopropyl ether Usage And Synthesis |
| Chemical Properties | colourless liquid |
| Uses | As solvent; fuel additive. |
| General Description | A clear colorless liquid with an ethereal odor. Flash point -18°F. Less dense than water. Vapors heavier than air. |
| Air & Water Reactions | Highly flammable. Slightly soluble in water. Form explosive peroxide in storage. A flask of Isopropyl ether was heated on a steam bath with gentle shaking when an explosion occurred. In a second instance, an explosion occurred after practically all the ether had been distilled, [MCA Guide for Safety(1972)]. |
| Reactivity Profile | Ethers, such as Isopropyl ether, can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert. Mixing Isopropyl ether in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid, [NFPA 1991]. |
| Hazard | Flammable, dangerous fire risk, explosive limits in air 1.4–21%. Toxic by inhalation, strong irritant. |
| Health Hazard | Inhalation causes anesthesia, nausea, headache, dizziness, and irritation of the eyes and nose. Contact of liquid with eyes causes only minor injury; repeated contact with skin will remove natural oils and may cause dermatitis. |
| Fire Hazard | Behavior in Fire: Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. Containers may explode when heated. |
| Purification Methods | Common impurities are water and peroxides [detected by the liberation of iodine from weakly acid (HCl) solutions of 2% KI]. Peroxides can be removed by shaking with aqueous Na2SO3 or with acidified ferrous sulfate (0.6g FeSO4 and 6mL conc H2SO4 in 110mL of water, using 5-10g of solution per L of ether), or aqueous NaBH4 solution. The ether is then washed with water, dried with CaCl2 and distilled. Alternatively, refluxing with LiAlH4 or CaH2, or drying with CaSO4, then passage through an activated alumina column, can be used to remove water and peroxides. Other dehydrating agents used with isopropyl ether include P2O5, sodium amalgam and sodium wire. (The ether is often stored in brown bottles, or in the dark, with sodium wire.) Bonner and Goishi (J Am Chem Soc 83 85 1961) treated isopropyl ether with dilute sodium dichromate/sulfuric acid solution, followed by repeated shaking with a 1:1 mixture of 6M NaOH and saturated KMnO4. The ether is washed several times with water, dilute aqueous HCl, and water, with a final washing with, and storage over, ferrous ammonium sulfate acidified with H2SO4. Blaustein and Gryder (J Am Chem Soc 79 540 1957), after washing with alkaline KMnO4, then water, treated the ether with ceric nitrate in nitric acid, and again washed it with water. Hydroquinone is added before drying with CaCl2 and MgSO4, and refluxing with sodium amalgam (108g Hg/100g Na) for 2hours under nitrogen. The distillate (nitrogen atmosphere) is made 2 x 10-5M in hydroquinone to inhibit formation of peroxides (which is negligible if the ether is stored in the dark). Catechol (pyrocatechol) and resorcinol are alternative inhibitors. [Beilstein 1 IV 1471.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- ISO-PROPYL ETHER 108-20-3 99%
- Inquiry
- 2024-11-05
-
VIP1年
- SS Reagents and Chemicals
- Diisopropyl Ether 108-20-3 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Pallav Chemicals And Solvents Pvt Ltd
- Di-Isoproply Ether 98% For Synthesis 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Nabariya Impex
- Di Isopropyl Ether 99%
- Inquiry
- 2024-10-23
-
VIP1年
- Wellchem Laboratories
- 108-20-3 99%
- Inquiry
- 2024-03-18
-
VIP1年
- Expresolv Limited
- Diisopropyl ether 98%
- Inquiry
- 2024-02-07
-
VIP1年
- Manas Petro Chem
- 108-20-3 98%
- Inquiry
- 2024-02-02
-
VIP1年
- Kairav Chemofarbe Industries Ltd. KCIL
- 108-20-3 Diisopropyl ether 98%
- Inquiry
- 2024-01-27
-
VIP1年
- Merck Ltd
- Diisopropyl ether 108-20-3 98%
- Inquiry
- 2024-01-17
-
VIP1年
- Otto Chemie Pvt Ltd
- Diisopropyl ether 98%
- Inquiry
- 2024-01-04
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
