

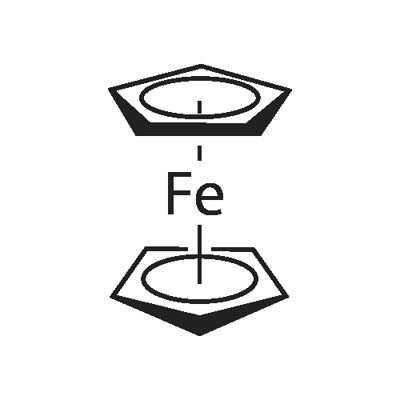
Ferrocene
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1g
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameFerrocene
- CAS No. 102-54-5
- EINECS No.203-039-3
- MFC10H10Fe
- MW186.03
- InChIKeySCFYIZNUVVGIOF-UHFFFAOYSA-N
- Appearancecrystalorange
- Melting point 172-174 °C (lit.)
- Boiling point 249 °C (lit.)
- Water Solubility practically insoluble
- storage temp. Store below +30°C.
- density 1.490
| Ferrocene Basic information |
| Product Name: | Ferrocene |
| Synonyms: | BIS(CYCLOPENTADIEN)IRON;BIS(CYCLOPENTADIENYL)IRON;BIS(CYCLOPENTADIENYL)IRON(+2);FERROCENE;IRON DICYCLOPENTADIENYL;di-2,4-cyclopentadien-1-yliron;DICYCLOPENTADIENYLIRON;DICYCLOPENTADIENYLIRON(II) |
| CAS: | 102-54-5 |
| MF: | C10H10Fe |
| MW: | 186.03 |
| EINECS: | 203-039-3 |
| Product Categories: | Organometallics;Classes of Metal Compounds;Fe (Iron) Compounds;Ferrocenes;Metallocenes;Transition Metal Compounds;metallocene |
| Mol File: | 102-54-5.mol |
 |
|
| Ferrocene Chemical Properties |
| Melting point | 172-174 °C(lit.) |
| Boiling point | 249 °C(lit.) |
| density | 1.490 |
| vapor pressure | 0.03 mm Hg ( 40 °C) |
| Fp | 100°C |
| storage temp. | Flammables area |
| form | crystal |
| color | orange |
| Water Solubility | practically insoluble |
| Sensitive | Air & Moisture Sensitive |
| Sublimation | 100 ºC |
| Merck | 14,4037 |
| Stability: | Stable at room temperature. Incompatible with strong oxidizing agents. Highly flammable. |
| CAS DataBase Reference | 102-54-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Ferrocene(102-54-5) |
| EPA Substance Registry System | Ferrocene(102-54-5) |
| Safety Information |
| Hazard Codes | F,Xn,N |
| Risk Statements | 11-22-51/53-2017/11/22 |
| Safety Statements | 61-22-24/25 |
| RIDADR | UN 1325 4.1/PG 2 |
| WGK Germany | 2 |
| RTECS | LK0700000 |
| TSCA | Yes |
| HazardClass | 4.1 |
| PackingGroup | II |
| HS Code | 29310095 |
| Hazardous Substances Data | 102-54-5(Hazardous Substances Data) |
| Ferrocene Usage And Synthesis |
| A kind of organic transition metal compound with aromatic property | Ferrocene is a kind of organic transition metal compound with aromatic nature. It is also called as dicyclopentadienyl iron. It contains a divalent iron cation and two cyclopentadienyl anions in its molecular structure. It is also the raw materials for production of ferrocenecarboxylic acid. In 1950s of the last century, people had first successfully produced it with the reaction between cyclopentadienyl magnesium bromide and anhydrous ferric chloride. At room temperature, it is orange needle crystal powder with a similar smell as camphor and belongs to non-polar compound. It is soluble in many organic solvents such as methanol, ethanol, ethyl ether, petroleum ether, gasoline, kerosene, diesel oil, methylene chloride, benzene, toluene, and xylene, Because of the large polarity of ethanol polarity, it is usually recommended to dissolve it with toluene. It is insoluble in water but soluble in concentrated sulfuric acid. It is also insoluble and doesn’t decompose in boiling caustic soda solution and hydrochloric acid. It molecule exhibits polarity with higher thermal stability, chemical stability and radiation resistance. It has wide range of application in industry, agriculture, medicine, aerospace, energy, environmental protection and other industries. Main applications are described below: (1)It can be used as the fuel saving smoke suppressants and anti-knock agent. For example, it can be used for the production of gasoline antiknock agent, the fuel catalyst of rocket propellant and also the solid fuels of aerospace. (2) It can be used as the catalyst such as catalyst for the production of ammonia, as the curing agent of silicone rubber; it can prevent the degradation of polyethylene by light; when applied to agricultural mulch, it can break its natural degradation without affecting the cultivation and fertilization within a certain time. (3) It can be used as a gasoline anti-knock agent. It can substitute gasoline toxic tetraethyl lead for being used as the anti-knock agent and for production of high-grade unleaded petrol in order to eliminate the contamination of the environment and poisoning to human body by discharge of fuel. (4) It can be used as radiation absorbers, heat stabilizers, light stabilizers, and smoke-retardants. (5) For the chemical properties, ferrocene is similar to aromatic compounds which is not prone to have addition reaction but prone to have electrophilic substitution reaction. It can also participate in metallization, acylation, alkylation, sulfonation, formylation and ligand exchange reaction, which can be used for production of derivative with a wide range of applications. The above information is edited by the Chemicalbook of Dai Xiongfeng. |
| Chemical Properties | It is an orange needles like crystal with its melting point being 172.5-173 ℃. It undergoes sublimation at temperature higher than 100 ℃. Its boiling point is 249 ℃. It is soluble in dilute nitric acid, concentrated sulfuric acid, benzene, ether, petroleum ether and tetrahydrofuran. It can generate bluish fluorescence-containing deep red solution in dilute nitric acid and concentrated sulfuric acid. It is insoluble in water, 10% sodium hydroxide and hot concentrated hydrochloric acid. In the boiling solution of these solvents, ferrocene is neither dissolved nor decomposed. It can be evaporated together with water vapor. It has a smell similar to camphor and is stable in air. It has a strong property of UV absorbing and has great thermostability which can withstand the heating at a temperature as high as 470 ℃. |
| Uses | Ferrocene can be used as the additives of rocket fuel, the antiknock agent of gasoline and the curing agent of rubber and silicone resin as well as the ultraviolet absorber. The Vinyl derivative of ferrocene can be subject to ethylenic polymerization to obtain the metal-containing polymers of carbon chain skeleton which can be used as the outer coating of spacecraft. It has been found early regarding the smoke abatement effect and combustion effect of ferrocene. No matter whether being supplied in solid fuel, liquid fuel or gas fuel, it can always exert this kind of effect and this effect is more significant especially for hydrocarbons with smoke upon burning. Supplement it into the gasoline has a very good anti-hunt effect. However, due to the deposition of the iron oxide in park plug which can negatively affect ignition, its application is limited, therefore, some people also apply de-iron mixture in order to reduce the deposition phenomenon of iron. Supplement of ferrocene to the kerosene or diesel oil, due to the absence of ignition device in the engine, will have fewer adverse effects on it. In addition to the smoke abatement and combustion facilitating effect in the combustion, it also has effect of promoting the conversion of carbon monoxide into carbon dioxide. In addition, it can improve the heat of combustion and increase power efficiency to achieve the effects of energy saving and air pollution reduction. Supplement of ferrocene to the boiler fuel oil can reduce tobacco production and nozzle deposition. Supplement of 0.1% ferrocene in diesel can remove smoke by 30-70%, save fuel by 10-14%, and increase the power by 10%. There are even many more reports regarding the application of ferrocene in solid fuel of rocket fuel. Moreover, there are even cases regarding supplying it in pulverized coal for smoke-reduction agent. Upon applying the polymer water as fuel, supplement of ferrocene can reduce the smoke by several times. It can also be used as the smoke-reducing additive. In addition to the above applications, ferrocene also has other applications. When used as iron fertilizer, it can facilitate the absorption of plants on iron and increase the iron content of crops. Moreover, its derivatives can be used as pesticides. Ferrocene also has a lot of applications in industry and organic synthesis. For example, its derivatives can be used as the antioxidant of rubber or polyethylene, the stabilizer of polyurea ester, the catalyst of isobutene spasm methylation, the decomposition catalyst of polymer peroxide, and for increasing the yield of para-chlorotoluene produced during the process of chlorination of toluene. In other areas, it can also be used as anti-load additive of lubricants as well as the promoting agent of abrasive materials. It can be used as catalyst and the antiknock additive of gasoline as well as energy-saving additives for combustion promotion and smoke removal; it can be applied to various kinds of fuels such as diesel, gasoline, heavy oil and coal. Supplement of 0.1% ferrocene into diesel can save fuel by 10-14%, increase the efficiency by 10-13%, and reduce the degree of smoke in exhaust by 30-80 ‰. In addition, addition of 0.3 ‰ to the heavy oil and addition of 0.2% ferrocene to coal can both decrease the fuel consumption rate while the degree of smoke is also reduced by 30%. Ferrocene is a kind of sandwich-containing metal compound. Ferrocene and its derivatives, because of their own characteristics such as hydrophobicity, bio-oxidability, aroma, stability, low resistance, biological activity, has a wide range of application such as catalyst, the antiknock additive of gasoline, high-temperature lubricant, the intermediates of high-temperature polymer and UV absorber. |
| Production method | It can be produced either by the heating reaction between iron powder and cyclopentadiene in nitrogen atmosphere of 300 ℃ or the reaction between anhydrous ferric chloride together with the sodium cyclopentadienyl in tetrahydrofuran. Alternatively, it can also produced by electrolytic synthesis method. Taking cyclopentadiene, ferrous chloride, and diethylamine as raw material for synthesis of ferrocene can operate according to the following protocol. Upon stirring, add anhydrous ferric chloride (FeCl3) in several times to the tetrahydrofuran solution; further add iron powder into it and have heating reflux for 4.5 h under the protection of nitrogen gas, resulting in ferrous chloride solution. Further remove tetrahydrofuran solvent by evaporation under reduced pressure to give nearly dry residue. Under ice-cooling condition, add the mixture of cyclopentadienyl and diethylamine and stir vigorously at room temperature for 6-8h; remove the excess amount of amine through evaporation under reduced pressure and extract the residue with petroleum ether under reflux. The extract is subject to immediate filtering; evaporate the solvent to obtain ferrocene crude product. Employ pentane or cyclohexane for recrystallization, or apply sublimation method for extract purified product with the yield of refined product being 73-84%. |
| Category | toxic substances |
| Toxicity grading | poisoning |
| Acute toxicity | Oral-rat LD50: 1320 mg/kg; Oral-Mouse LD50: 832 mg/kg. |
| Flammability and hazard characteristics | flammable with the combustion generating iron-containing acrid smoke |
| Storage properties | warehouse: ventilated, low temperature and dry; Store it separately from oxidants. |
| Extinguishing agent | Water, carbon dioxide, dry, sandy soil. |
| Professional standards | TWA 10 mg/m³; STEL 20 mg/m3 |
| Chemical Properties | Orange, crystalline solid; camphor-like odor. Insoluble in water; soluble in benzene, ether, and alcohol. Iron content 29.4–30.6%. |
| Uses | Antiknock additive for gasoline; catalyst. |
| Definition | A coordination compound of ferrous iron and two molecules of cyclopentadiene in which the organic portions have typically aromatic chemical properties. Its activity is intermediate between phenol and anisole. The first compound shown to have the “sandwich |
| General Description | Orange crystalline solid or orange-yellow powder. Sublimes above 212°F. Camphor odor. |
| Air & Water Reactions | Sensitive to prolonged exposure to air and may be sensitive to light. Insoluble in water. |
| Reactivity Profile | Ferrocene reacts violently with tetranitromethane. . Contact of tetranitromethane with Ferrocene under various conditions leads to violent explosion, [Trans. Met. Chem., 1979, 4, 207-208]. |
| Hazard | Moderate fire risk. Evolves toxic products on decomposition and heating. |
| Fire Hazard | Flash point data for Ferrocene are not available. Ferrocene is probably combustible. |
| Safety Profile | Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Flammable; reacts violently with NH4ClO4. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Purification Methods | Purify it by crystallisation from pentane or cyclohexane (also *C6H6 or MeOH can be used). It is moderately soluble in Et2O and sublimes readily above 100o. Crystallisation from EtOH gave material m 172.5-173o. [Wilkinson Org Synth Coll Vol IV 473 1963, Miller J Chem Soc 632 1952.] It has also been crystallised from methanol and sublimed in vacuo. [Saltiel et al. J Am Chem Soc 109 1209 1987, Beilstein 16 IV 1783.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Pallav Chemicals And Solvents Pvt Ltd
- Ferrocene 98% For Synthesis 102-54-5 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Dr. Silviu Pharmachem Pvt., Ltd.
- 102-54-5 / 55404-68-7 98%
- Inquiry
- 2024-01-19
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
