

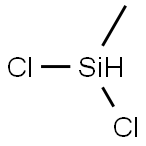
Dichloromethylsilane
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameDichloromethylsilane
- CAS No. 75-54-7
- EINECS No.200-877-1
- MFCH4Cl2Si
- MW115.03
- InChIKeyNWKBSEBOBPHMKL-UHFFFAOYSA-N
- AppearanceLiquidColorless
- Melting point -93 °C
- density 1.105 g/mL at 25 °C(lit.)
- storage temp. 2-8°C
- Boiling point 41 °C(lit.)
- Water Solubility DECOMPOSES
| Dichloromethylsilane Basic information |
| Product Name: | Dichloromethylsilane |
| Synonyms: | CM8750;dichlorohydridomethylsilicon;dichloromethyl-silan;dichloromethylsilane,[flammableliquid];Methyldichlorosilan;Methyl-dichlorsilan;methyl-dichlorsilan(czech);monomethyldichlorosilane |
| CAS: | 75-54-7 |
| MF: | CH4Cl2Si |
| MW: | 115.03 |
| EINECS: | 200-877-1 |
| Product Categories: | Chloro;Dichlorosilanes;Si (Classes of Silicon Compounds);Si-Cl Compounds;Si-H Compounds;Chloro Silanes;organosilicon compounds |
| Mol File: | 75-54-7.mol |
 |
|
| Dichloromethylsilane Chemical Properties |
| Melting point | -93 °C |
| Boiling point | 41 °C(lit.) |
| density | 1.105 g/mL at 25 °C(lit.) |
| vapor density | 4 (vs air) |
| vapor pressure | 6.79 psi ( 20 °C) |
| refractive index | n20/D 1.398(lit.) |
| Fp | −26 °F |
| storage temp. | 2-8°C |
| solubility | DECOMPOSES |
| form | Liquid |
| color | Colorless |
| explosive limit | >55% |
| Water Solubility | DECOMPOSES |
| Sensitive | Air & Moisture Sensitive |
| InChIKey | NWKBSEBOBPHMKL-UHFFFAOYSA-N |
| CAS DataBase Reference | 75-54-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, dichloromethyl-(75-54-7) |
| EPA Substance Registry System | Silane, dichloromethyl-(75-54-7) |
| Safety Information |
| Hazard Codes | F,T,C |
| Risk Statements | 11-14/15-28-34-37-35-20/22 |
| Safety Statements | 16-26-36/37/39-43-45-7/8-30 |
| RIDADR | UN 1242 4.3/PG 1 |
| WGK Germany | 1 |
| RTECS | VV3500000 |
| F | 10-21 |
| TSCA | Yes |
| HazardClass | 4.3 |
| PackingGroup | I |
| Hazardous Substances Data | 75-54-7(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| Methyldichlorosilane | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Dichloromethylsilane Usage And Synthesis |
| Chemical Properties | Colorless clear liquid |
| Uses | Manufacture of siloxanes. |
| General Description | A colorless fuming liquid with a pungent odor. Flash point: -26°F; Boiling point: 41°C (106°F) . Vapors heavier than air. Vapor and liquid may cause burns. Denser than water and decomposed by water to form hydrochloric acid, a corrosive material. |
| Reactivity Profile | Chlorosilanes, such as Dichloromethylsilane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases. Impact of dichlorosilane causes mixtures to burst into flame. This is the case, when the dichlorosilane and most likely related substances are mixed with oxidants such as potassium permanganate, lead oxide, copper oxide, or silver oxide, even under inert gas atmosphere [Bretherick, 1995, pg. 193]. With Dichloromethylsilane, toxic hydrogen chloride and phosgene gases may be formed when burned. |
| Hazard | Flammable, dangerous fire risk. Very toxic. |
| Health Hazard | Inhalation causes irritation of respiratory tract; heavy exposure can cause pulmonary edema. Contact of liquid with skin or eyes causes severe burns. Ingestion causes burns of mouth and stomach. |
| Safety Profile | Moderately toxic by inhalation. Corrosive. A severe irritant to skin, eyes, and mucous membranes. Ignites spontaneously in air. A very dangerous fire hazard when exposed to heat or flame. Forms impact-sensitive explosive mixtures with potassium permanganate, lead(Ⅱ) oxide, lead(Ⅳ) oxide, copper oxide, silver oxide. To fight fire, use water, foam, CO2, mist. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROSILANE. |
| Purification Methods | Impurities are generally other chloromethyl silanes. Distil it through a conventional Stedman column (p 11) of 20 theoretical plates or more. It should be protected from H2O by storing over P2O5. [Stck & Somieski Chem Ber 52 695 1919, Sauer J Am Chem Soc 68 962 1946, Beilstein 4 IV 4096.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
