

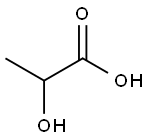
Lactic acid
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product Name Lactic acid
- CAS No. 50-21-5
- EINECS No.200-018-0
- MFC3H6O3
- MW90.08
- InChIKeyJVTAAEKCZFNVCJ-UHFFFAOYSA-N
- AppearancesyrupColorless to yellow
- storage temp. 2-8°C
- Boiling point 122 °C/15 mmHg (lit.)
- Melting point 18°C
- density 1.209 g/mL at 25 °C (lit.)
- Water Solubility SOLUBLE
AD68
| Lactic acid Basic information |
| Product Name: | Lactic acid |
| Synonyms: | FEMA 2611;DL-ALPHA-HYDROXYPROPIONIC ACID;(+/-)-LACTIC ACID;LACTIC ACID;LACTIC ACID, RACEMIC;2-HYDROXYPROPIONIC ACID, RACEMIC;(+/-)-2-HYDROXYPROPIONIC ACID;2-HYDROXYPROPIONIC ACID |
| CAS: | 50-21-5 |
| MF: | C3H6O3 |
| MW: | 90.08 |
| EINECS: | 200-018-0 |
| Product Categories: | FOOD ADDITIVES;Plant Growth Regulator;Food & Feed ADDITIVES;ACS GradeChiral Building Blocks;Carboxylic Acids;Essential Chemicals;Organic Building Blocks;Routine Reagents;Food & Flavor Additives;Building Blocks;C1 to C5;Carbonyl Compounds;Carboxylic Acids;Chemical Synthesis;Organic Building Blocks;Food additive and acidulant |
| Mol File: | 50-21-5.mol |
 |
|
| Lactic acid Chemical Properties |
| Melting point | 18°C |
| Boiling point | 122 °C15 mm Hg(lit.) |
| alpha | -0.05 º (c= neat 25 ºC) |
| density | 1.209 g/mL at 25 °C |
| refractive index | n20/D 1.4262 |
| FEMA | 2611 | LACTIC ACID |
| Fp | >230 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with water and with ethanol (96 per cent). |
| form | syrup |
| pka | 3.08(at 100℃) |
| color | Colorless to yellow |
| Water Solubility | SOLUBLE |
| Merck | 14,5336 |
| BRN | 1209341 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 50-21-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanoic acid, 2-hydroxy-(50-21-5) |
| EPA Substance Registry System | Propanoic acid, 2-hydroxy-(50-21-5) |
| Safety Information |
| Hazard Codes | Xi,C |
| Risk Statements | 38-41-34-37/38 |
| Safety Statements | 26-39-45-36/37/39 |
| RIDADR | 3265 |
| WGK Germany | 2 |
| RTECS | OD2800000 |
| F | 3 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| Hazardous Substances Data | 50-21-5(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| 2-Hydroxypropanoic acid | English |
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| Lactic acid Usage And Synthesis |
| Chemical Properties | Lactic acid, CH3CHOHCOOH, also known as 2-hydroxypropanoic acid, is a hygroscopic liquid that exists in three isometric forms. I-lactic acid is found in blood and animal tissue as a product of glucose and glycogen metabolism. d-Iactic acid is obtained by fermentation of sucrose (corn refining), The racemic mixture is present in foods prepared by bacterial fermentation or prepared synthetically. Lactic acid is soluble in water,alcohol,and ether. It is used as a solvent, in manufacturing confectionery, and in medicine. |
| Uses | Prostaglandin E1 analogue |
| Uses | lactic acid (sodium lactate) is a multi-purpose ingredient used as a preservative, exfoliant, moisturizer, and to provide acidity to a formulation. In the body, lactic acid is found in the blood and muscle tissue as a product of the metabolism of glucose and glycogen. It is also a component of the skin’s natural moisturizing factor. Lactic acid has better water intake than glycerin. Studies indicate an ability to increase the water-retention capacity of the stratum corneum. They also show that the pliability of the stratum corneum layer is closely related to the absorption of lactic acid; that is, the greater the amount of absorbed lactic acid, the more pliable the stratum corneum layer. Researchers report that continuous use of preparations formulated with lactic acid in concentrations ranging between 5 and 12 percent provided a mild to moderate improvement in fine wrinkling and promote softer, smoother skin. Its exfoliating properties can help in the process of removing excess pigment from the surface of the skin, as well as improving skin texture and feel. Lactic acid is an alpha hydroxy acid occurring in sour milk and other lesser-known sources, such as beer, pickles, and foods made through a process of bacterial fermentation. It is caustic when applied to the skin in highly concentrated solutions. |
| Definition | ChEBI: A 2-hydroxy monocarboxylic acid that is propanoic acid in which one of the alpha-hydrogens is replaced by a hydroxy group. |
| General Description | A colorless to yellow odorless syrupy liquid. Corrosive to metals and tissue. Used to make cultured dairy products, as a food preservative, and to make chemicals. |
| Air & Water Reactions | Soluble in water. |
| Reactivity Profile | Lactic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Lactic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. Slowly corrodes most metals [USCG, 1999]. |
| Health Hazard | Inhalation of mist causes coughing and irritation of mucous membranes. Ingestion, even of diluted preparations, has a corrosive effect on the esophagus and stomach. Contact with more concentrated solutions can cause severe burns of skin or eye. |
| Fire Hazard | Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- LACTIC ACID 99%
- Inquiry
- 2024-11-05
-
VIP1年
- Prakash Chemicals Agencies
- 50-21-5 / 598-82-3 99%
- Inquiry
- 2024-02-14
-
VIP1年
- Enzyme BioScience pvt Ltd
- 50-21-5 / 598-82-3 Lactic acid 99%
- Inquiry
- 2024-02-08
-
VIP1年
- Jiaan Biotech
- Lactic acid 50-21-5 / 598-82-3 98%
- Inquiry
- 2024-01-27
-
VIP1年
- Merck Ltd
- Lactic acid 98%
- Inquiry
- 2024-01-17
-
VIP1年
- Prathista Industries Limited
- 50-21-5 / 598-82-3 99%
- Inquiry
- 2024-01-16
-
VIP1年
- HRV Global Life Sciences
- 50-21-5 / 598-82-3 Lactic acid 99%
- Inquiry
- 2024-01-12
-
VIP1年
- BASF India Limited
- Lactic acid 50-21-5 / 598-82-3 98%
- Inquiry
- 2024-01-05
-
VIP1年
- JSK Chemicals
- Lactic acid, 85% 99%
- Inquiry
- 2023-12-22
-
VIP1年
- UNILOSA INTERNATINAL PRIVATE LIMITED
- 50-21-5 99%
- Inquiry
- 2023-12-16
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
