

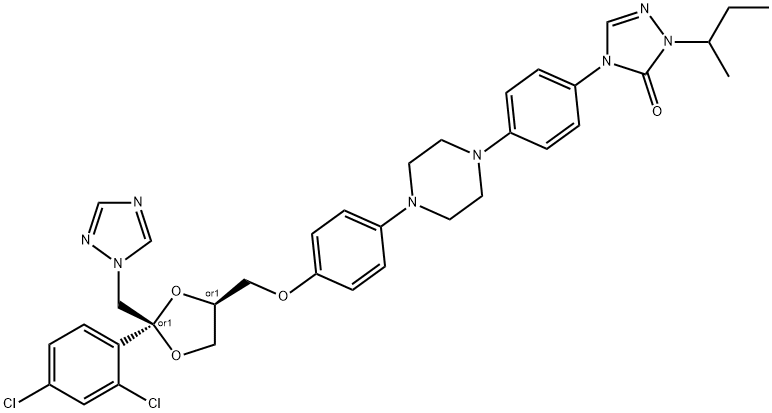
Itraconazole
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameItraconazole
- CAS No. 84625-61-6
- EINECS No.617-596-9
- MFC35H38Cl2N8O4
- MW705.63
- AppearanceWhite powderwhite
- storage temp. 2-8°C
- Melting point 166°C
- Water Solubility Insoluble in water. Solube in chloroform at 50 mg/ml. Slightly soluble in ethanol or methanol
- Boiling point 850.0±75.0 °C(Predicted)
- density 1.27 g/cm3
AD68
| Itraconazole Basic information |
| Product Name: | Itraconazole |
| Synonyms: | SPORANOX;R51211;ORICONAZOLE;TRIASPORIN;-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-1-piperazinyl)phenyl)-2,4-dihyd;3h-1,2,4-triazol-3-one,4-(4-(4-(4-((2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol;ro-2-(1-methylpropyl)-;ITRACONAZOLE-D3 |
| CAS: | 84625-61-6 |
| MF: | C35H38Cl2N8O4 |
| MW: | 705.63 |
| EINECS: | 1806241-263-5 |
| Product Categories: | Active Pharmaceutical Ingredients;Antibiotic Explorer;Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Antifungal;Metabolite Reference Standard;API Reference Standard Free Base API;Related Substance;API |
| Mol File: | 84625-61-6.mol |
 |
|
| Itraconazole Chemical Properties |
| Melting point | 166°C |
| Fp | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | chloroform: 50 mg/mL, clear, colorless |
| pka | 3.7(at 25℃) |
| color | white |
| Water Solubility | Insoluble in water. Solube in chloroform at 50 mg/ml. Slightly soluble in ethanol or methanol |
| Merck | 14,5245 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 84625-61-6(CAS DataBase Reference) |
| Safety Information |
| Hazard Codes | Xi,Xn,T,F |
| Risk Statements | 36/37/38-36/38-22-39/23/24/25-23/24/25-11 |
| Safety Statements | 22-26-36-45-36/37-16 |
| RIDADR | UN 3286 8(6.1)(3) / PGII |
| WGK Germany | 3 |
| RTECS | XZ5481000 |
| Toxicity | LD50 (14 day) in mice, rats, dogs (mg/kg): >320, >320, >200 orally (Van Cauteren) |
| Itraconazole Usage And Synthesis |
| Broad - spectrum antifungal agents | Itraconazole is an artificially synthetic clotrimazole, being a broad-spectrum synthetic antifungal agent. Its antimicrobial spectrum and antimicrobial mechanism is similar to clotrimazole, but has strong antibacterial activity against Aspergillus. It exerts its anti-fungal effect through changing the fungal cell membrane permeability with antibacterial activity against superficial and deep fungal pathogens. Its antibacterial spectrum is broader and stronger than ketoconazole, being able to inhibit the ergosterol synthesis of fungal cell membrane, thus playing the antifungal effect. This product is suitable for the treatment of Dermatophytes (Trichophyton, Microsporum, Flocculent Epidermophyton), Yeast [Cryptococcus neoformans, Pityrosporum, Candida (including Candida albicans, Candida glabrata and candida krusei)], Aspergillus, Histoplasma, Paracoccidioides brasiliensis, Sporothrix schenckii, Hormodendrum, Cladosporium, Blastomyces dermatitidis and various kinds of other types of yeasts and fungi. Itraconazole is not able to inhibit the growth of Rhizopus and Mucor. One nitrogen atom contained in the itraconazole molecule can bind to the ferrous ion on the heme of the P450 molecule in the fungal cell, thereby inhibiting the P450 hydroxylase-catalyzed de-methylation of lanosterol, Sterol to ergosterol conversion blocked, lanosterol and other precursors accumulation, membrane chemical composition changes, membrane associated enzyme dysfunction, increased permeability, intracellular fluid spill, so as to achieve the inhibition and sterilization. The combination of this product with the fungus P450 system is quite strong while its combination with the mammalian P450 system is weak, so that the toxicity of drugs on human is greatly reduced. Clinical Itraconazole is mainly used for the treatment of systemic deep fungal infections caused by systems such as blastomycosis, histoplasmosis, coccidioidomycosis, chromoblastomycosis, sporotrichosis and coccidioidomycosis. It can also be used for the treatment of candidiasis and aspergillosis. The above information is compiled and edited by Tongtong from Chemicalbook. |
| Pharmacokinetics | Itraconazole, when taken immediately after the meal, can give the highest bioavailability. After (4.6 ± 1.3) hours of oral administration of 200 mg of itraconazole, the plasma concentration can reach peak with the plasma concentration being (0.32 ± 0.16) mg/mL. The product has a plasma protein binding rate of 99.8% with the whole blood concentration being about 60% of the plasma concentration. The drug concentration in the lung, kidney, liver, bone, stomach, spleen and muscle was found to be 2 to 3 times as high as that in the corresponding plasma concentration. In keratin-rich tissues, especially in the skin, the concentration is four times as high as that in the plasma concentration while the drug clearance is associated with the process of epidermal regeneration. If withdraw the drug after continuous medication of 4 weeks, the plasma concentration become undetectable after 7 days while drug contained in the skin can be still maintained in the drug treatment concentration for 2 to 4 weeks. After a week of starting the treatment, it can be measured of itraconazole in the keratin. At the end of the 3-month treatment course, the drug concentration can be maintained for at least 6 months. This product is present in sebum with a small amount also existing in sweat. Itraconazole is also concentrated in areas susceptible to fungal infection. The duration of the treatment concentration in the vaginal tissue is: 200mg once per day for 3 days, last for 2 days; 200mg 2 times a day for 1 day, can last 3 days. Itraconazole is mainly metabolized in the liver, producing large amounts of metabolites. One of them is hydroxylated itraconazole; in vitro study has found that it has a similar antifungal activity with this product. The level of antifungal drug measured by bioanalytical method is three times as high as the level measured by high-pressure liquid chromatography analysis. This product was biphasic in plasma clearance with the terminal half-life of (23.8 ± 4.7) hours. The dosage of the prototype drug through fecal excretion accounts for about 3 to 18% of the total dose. The prototype drug via renal excretion is less than 0.03% of the dose with about 35% being subject to urine excretion in the form of metabolites within a week. |
| Clinical application and indications | Itraconazole is applicable for the treatment of the following diseases: 1, it can be used for the treatment of systemic fungal infections, such as aspergillosis, candidiasis, cryptococcosis (including cryptococcal meningitis), histoplasmosis, sporotrichosis, Paracoccidioides brasiliensis disease, blastomycosis and many other kinds of rare systemic or tropical fungal diseases. 2, it can be used for the treatment of the candidiasis infection occurred in the mouth, throat (foreign data), esophageal (foreign data) and vulvovagina, and fungal conjunctivitis, fungal keratitis. 3, it can be used for the treatment of superficial fungal infections, such as hand, foot and ringworm, tinea corporis, tinea corporis, tinea versicolor and so on. 4, it can be used for the treatment of dermatophytes and (or) yeast-induced onychomycosis. |
| Dosage and Usage | Capsules: In order to achieve optimal absorption, itraconazole capsules should be administered immediately after a meal and the capsule must be swallowed. 1. Candida vaginitis: 200mg each time, twice a day, a course of treatment for 1 day or 200mg, once the course of treatment of 3 days; 2. Tinea versicolor: 200mg each time, once a day with the course of treatment of 7 days ; 3. Dermatomycosis: 100 mg each time, once a day with the course of treatment of 15 days; high-degree keratinized area (such as foot bottom ringworm, hand tinea) need: extended treatment of 15 days; 4 Oral candidiasis: 100mg each time, once a day with the course of treatment of 15 days; 5. Fungal keratitis: 200 mg each time, once a day with the course of treatment of 21 days; 6. For some immunodeficiency patients, such as leukemia (such as leukemia), AIDs and organ transplanted patients, application of itraconazole capsules for the treatment of fungal infections can get reduced oral bioavailability; in this case, the dose can be doubled. 7. Onychomycosis: 1) shock treatment: 200 mg each time, twice per day with continuous one week as a shock treatment. For the treatment of nail infections, it is recommended to adopt two shock treatment courses with each course having a gap of 3 weeks; for the treatment of toenail infection, it is recommended to adopt three shock treatment courses. Each treatment course has a gap of 3 weeks. 2) Or adopt continuous treatment: 200 mg each time, once a day, continue for three months. This product is removed more slowly in the skin and keratin tissue than removed in the plasma. Therefore, for skin infections, 2 to 4 weeks after stopping the drug can achieve the most ideal clinical and mycological efficacy. For the treatment of onychomycosis, people can achieve the best clinical and mycological efficacy after 6~9 months of stopping the drugs. Injection: during the first two days, give itraconazole injection 2 times a day, later change to once per day. Treatment protocol in Day 1 and 2: 2 times per day; adopt intravenous infusion of 200 mg itraconazole 1 hour each time. From day 3: perform once per day with intravenously infusion of 200 mg of itraconazole for 1 hour each time. The safety for intravenous administration of more than 14 days is not clear. |
| Medicine interactions | 1. Enzyme-induction Drugs: for example, rifampicin and phenytoin can significantly reduce the oral bioavailability of this product. Therefore, upon administration together with the enzyme-induction drugs, we should monitor the plasma concentration of the goods. 2 In vitro studies have showed that there was no interaction between itraconazole and imipramine, propranolol, diazepam, cimetidine, indomethacin, methotrexate and sulfadimidine regarding to the plasma protein binding. 3. It has been reported that this product, when exceeding the recommendation dosage, has interaction with cyclosporine A, astemizole and terfenadine. If these drugs are administrated together with this product, we should reduce their doses. 4. This product has been reported have interaction with warfarin and digoxin. Therefore, if these drugs are administrated together with this product, we should reduce their doses. 5. It has been not observed of the interaction of this product with AZT (zidovudine). 6. It has been not observed of the induction effects of itraconazole on the metabolism of ethinyl estradiol and norethindrone. Adverse reactions It is commonly observed of gastrointestinal discomfort, such as anorexia, nausea, abdominal pain and constipation. Less common side effects include headache, elevated reversible aminotransferase, menstrual disorders, dizziness and allergic reactions (such as itching, erythema, wheal and angioedema). There are a few cases that have reported Stevens-Johnson syndrome (severe erythema multiforme). The majority of patients who have had a potential pathologic change and are undergoing multiple drug regimens can get symptoms such as hypokalemia, edema, hepatitis, and alopecia during long-term treatment with itraconazole. There are individual cases that have reported the peripheral neuropathy, but whether it is related to taking itraconazole is uncertain. Stability This product appears as colorless to yellowish solution, should be stored at room temperature, being avoid of light and freezing. Before application, we should check whether there are particles emerging and whether there is discoloration. After the preparation of the solution, it can be stored for 48 h at room temperature or refrigerated and dark conditions. |
| Clinical evaluation | Tinea corporis, tinea corporis and tinea pedis can obtain the cure rate or significant efficiency of 80% or more. The cure rate of Pityriasis versicolor can be over 90%. The negative conversion ratio of vaginal candidiasis fungus can reach 80%. 80% of the cases of blastomycosis, sporotrichosis, and histoplasmosis can get clinically cured or markedly. |
| Precautions | 1. It is recommended to check liver function in patients who have continued medication for more than 1 month and who have develop anorexia, nausea, vomiting, fatigue, abdominal pain or dark urine during the treatment. If abnormal, they should stop medication. 2. Itraconazole is mostly subject to the liver metabolism, and thus patients of abnormal liver function should take with caution (unless the need for treatment is higher than the risk of liver damage). 3. Stop the treatment immediately upon the occurrence of neurological symptoms. 4. For patients with renal insufficiency, the excretion of the goods slows down. It is recommended to monitor the plasma concentration of this product to determine the appropriate dose. |
| Drug overdose | Once occurs, we should take supportive therapy, including gastric lavage. Itraconazole can’t be removed by hemodialysis with no special antidote. |
| Chemical properties | It is crystallized from toluene with the melting point of 166.2 °C and PKa of 3.7. It is almost insoluble in water and the dilute acid solution. Acute toxicity LD50 (14 days) mice, rats, dogs (mg/kg):> 320,> 320,> 200 orally. |
| Uses | The imidazole antifungal agents for the triazole ring, through inhibiting the activation of cytochrome P-450, including oxidase and peroxidase, disable the 14, α-methyl sterols to be dehydrogenated and be converted to ergosterol, so that the growth and proliferation of fungi are inhibited. They have strong lipophilicity and penetrate through the biofilm to inhibit the binding of the membrane to the fungus. The antibacterial spectrum is similar to ketoconazole. It is effective in the treatment of superficial fungal diseases such as vaginal and oral candidiasis as well as skin mycosis. Furthermore, it is expected to become the highly-efficient and safe drugs for the treatment of deep fungal infections such as cryptococcal encephalitis (AIDS). It is used for the treatment of vulvovaginal candidiasis, pityriasis and skin fungal disease pityriasis caused by sensitive fungi. It can be used for the synthesis of broad-spectrum azole antifungal agents for the treatment of systemic infection caused by deep fungus. It can also be used for the treatment of candidiasis and aspergillosis. |
| Production method | Starting from m-dichlorobenzene, compound (I) can be obtained through a reaction similar to ketoconazole. The compound (I) is further condensed with 1-acetyl-4-(4-hydroxyphenyl) piperazine, followed by hydrolysis to remove the acetyl group, condensation with p-nitrochlorobenzene, hydrogenation reduction, chloroformate esterification, and reaction with hydrazine hydrate. After cyclization and alkylation, itraconazole is finally obtained. The intermediate 1-acetyl-4-(4-hydroxyphenyl) piperazine can use piperazine as the starting material and prepared with the following reactions. |
| Chemical Properties | Off-White Crystalline Solid |
| Uses | vitamin, enzyme cofactor |
| Uses | Anti-infective |
| Uses | Anti Fungal. Used in the treatment of stomach upset/ indigestion and other gastrointestinal conditions |
| Uses | For the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: pulmonary and extrapulmonary blastomycosis, histoplasmosis, aspergillosis, and onychomycosis. |
| Uses | A traizole antifungal agent |
| Uses | An orally active antimycotic structurally related to Ketoconazole. Antifungal |
| Brand name | Sporanox (Janssen). |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- SETV ASRV LLP
- 84625-61-6 95-99 %
- Inquiry
- 2024-09-11
-
VIP1年
- AKASH PHARMA EXPORTS
- Itraconazole 99%
- Inquiry
- 2024-05-16
-
VIP1年
- Gangwal Healthcare Pvt Ltd
- 84625-61-6 98%
- Inquiry
- 2024-04-27
-
VIP1年
- Sibram Pharmaceutical
- 84625-61-6 99%
- Inquiry
- 2024-03-16
-
VIP1年
- Sri Sai Chandana API Pvt Ltd
- Itraconazole 84625-61-6 98%
- Inquiry
- 2024-03-15
-
VIP1年
- Apicore Pharmaceuticals Pvt Ltd
- Itraconazole 98%
- Inquiry
- 2024-03-07
-
VIP1年
- Vaasavaa Pharmaceuticals (P) Ltd
- 84625-61-6 98%
- Inquiry
- 2024-03-07
-
VIP1年
- Mylan Laboratories Ltd
- 84625-61-6 Itraconazole 98%
- Inquiry
- 2024-03-06
-
VIP1年
- SMS Lifesciences India Ltd (MAHI Drugs Pvt Ltd)
- Itraconazole 98%
- Inquiry
- 2024-02-28
-
VIP1年
- ProVentus Life Sciences Pvt Ltd
- 84625-61-6 98%
- Inquiry
- 2024-02-28
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
