

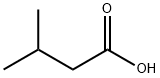
Isovaleric acid
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameIsovaleric acid
- CAS No. 503-74-2
- EINECS No.207-975-3
- MFC5H10O2
- MW102.13
- InChIKeyGWYFCOCPABKNJV-UHFFFAOYSA-N
- AppearanceLiquidClear colorless to slightly yellow
- Water Solubility 25 g/L (20 ºC)
- Melting point -29 °C (lit.)
- density 0.925 g/mL at 20 °C (lit.)
- Boiling point 175-177 °C (lit.)
- storage temp. Store below +30°C.
AD68
| Isovaleric acid Basic information |
| Product Name: | Isovaleric acid |
| Synonyms: | 3-METHYLBUTANOIC ACID;3-METHYLBUTYRIC ACID;AKOS BBS-00003796;RARECHEM AL BO 0154;3-Methylbuttersαure;3-methylbutyrate;3-methyl-butyricaci;3-Methyl-n-butyricacid |
| CAS: | 503-74-2 |
| MF: | C5H10O2 |
| MW: | 102.13 |
| EINECS: | 207-975-3 |
| Product Categories: | Pharmaceutical Raw Materials;Artemisia vulgaris;Building Blocks;C1 to C5;Carbonyl Compounds;Carboxylic Acids;Carthamus tinctorius (Safflower oil);Chemical Synthesis;Humulus lupulus (Hops);Hypericum perforatum (St John′;Nutrition Research;Organic Building Blocks;Panax ginseng;Phytochemicals by Plant (Food/Spice/Herb);s wort) |
| Mol File: | 503-74-2.mol |
 |
|
| Isovaleric acid Chemical Properties |
| Melting point | -35 °C |
| Boiling point | 176 °C |
| density | 0.926 |
| vapor pressure | 0.38 mm Hg ( 20 °C) |
| refractive index | n20/D 1.403(lit.) |
| FEMA | 3102 | ISOVALERIC ACID |
| Fp | 159 °F |
| storage temp. | Store below +30°C. |
| solubility | 48g/l |
| pka | 4.77(at 25℃) |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| PH | 3.1 (10g/l, H2O, 25℃) |
| explosive limit | 1.5-6.8%(V) |
| Water Solubility | 25 g/L (20 ºC) |
| Merck | 14,5231 |
| BRN | 1098522 |
| CAS DataBase Reference | 503-74-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanoic acid, 3-methyl-(503-74-2) |
| EPA Substance Registry System | Butanoic acid, 3-methyl-(503-74-2) |
| Safety Information |
| Hazard Codes | C,T |
| Risk Statements | 34-24-22 |
| Safety Statements | 26-36/37/39-45-38-28A |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 1 |
| RTECS | NY1400000 |
| F | 13 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| Hazardous Substances Data | 503-74-2(Hazardous Substances Data) |
| Toxicity | LD50 i.v. in mice: 1120±30 mg/kg (Or, Wretlind) |
| MSDS Information |
| Provider | Language |
|---|---|
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Isovaleric acid Usage And Synthesis |
| Chemical Properties | clear colorless to slightly yellow liquid |
| Definition | ChEBI: A C5, branched-chain saturated fatty acid. |
| Uses | In flavors, perfumes, manufacture of sedatives. |
| General Description | Isovaleric acid is a colorless liquid with a penetrating odor. Isovaleric acid is slightly soluble in water. Isovaleric acid is corrosive to metals and to tissue. |
| Air & Water Reactions | Isovaleric acid is slightly soluble in water. |
| Reactivity Profile | Isovaleric acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Isovaleric acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. |
| Hazard | Strong irritant to tissue. |
| Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
| Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Otto Chemie Pvt Ltd
- Isovaleric acid 98%
- Inquiry
- 2024-01-04
-
VIP1年
- JSK Chemicals
- Isovaleric acid 503-74-2 99%
- Inquiry
- 2023-12-22
-
VIP1年
- Ishant Polychem
- 1468-39-9 Isovaleric anhydride 98%
- Inquiry
- 2024-02-15
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
