

Silicon dioxide
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameSilicon dioxide
- CAS No. 7631-86-9
- EINECS No.231-545-4
- MFO2Si
- MW60.08
- AppearancesuspensionWhite to yellow
- Boiling point >100 °C(lit.)
- Melting point >1600 °C(lit.)
- density 2.2-2.6 g/mL at 25 °C
- Water Solubility insoluble
- storage temp. 2-8°C
AD68
| Silicon dioxide Basic information |
| Product Name: | Silicon dioxide |
| Synonyms: | SILICA GEL 60 PF254 FOR PREPARATIVE LAYE;LICHROSORB SI 100 (10 MYM) 10 G;TLC-SILICA GEL 60 GF254 MEAN PARTICLE SI;LICHROSORB SI 100 (10 MYM) 100 G;SEA SAND EXTRA PURE 5 KG;SILICA GEL 60 GF254 FOR THIN-LAYER CHROM;SILICA GEL 60 PF254 + 366 FOR PREPARATIV;SEA SAND EXTRA PURE 25 KG |
| CAS: | 7631-86-9 |
| MF: | O2Si |
| MW: | 60.08 |
| EINECS: | 231-545-4 |
| Product Categories: | #N/A;Siloxanes;Inorganics;Silica Gels;organofunctional catalyst support;metals scavenging agent;molecular sieves;metal oxide;Mesoporous Materials;Ceramic Science;Materials Science;Metal &;Nanomaterials;New Products for Materials Research and Engineering |
| Mol File: | 7631-86-9.mol |
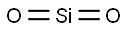 |
|
| Silicon dioxide Chemical Properties |
| Melting point | >1600 °C(lit.) |
| Boiling point | >100 °C(lit.) |
| density | 2.2-2.6 g/mL at 25 °C |
| refractive index | 1.46 |
| Fp | 2230°C |
| storage temp. | Refrigerator (+4°C) |
| solubility | Practically insoluble in water and in mineral acids except hydrofluoric acid. It dissolves in hot solutions of alkali hydroxides. |
| form | suspension |
| color | White to yellow |
| PH | 5-8 (100g/l, H2O, 20℃)(slurry) |
| Water Solubility | insoluble |
| Sensitive | Hygroscopic |
| Merck | 14,8493 |
| Stability: | Stable. |
| CAS DataBase Reference | 7631-86-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Silicon(iv) oxide(7631-86-9) |
| EPA Substance Registry System | Silica(7631-86-9) |
| Safety Information |
| Hazard Codes | Xn,Xi |
| Risk Statements | 36/37/38-36/37-22-43-52/53-36/38 |
| Safety Statements | 26-37/39-36-36/37/39-36/37-61 |
| WGK Germany | 1 |
| RTECS | VV7310000 |
| TSCA | Yes |
| HS Code | 28112200 |
| MSDS Information |
| Provider | Language |
|---|---|
| Silica | English |
| Silicon dioxide Usage And Synthesis |
| General Description | Silicon dioxide occurs almost everywhere on earth. It is one of the most important and abundant oxides on earth, constituting about 60% weight of the earth’s crust as silica itself or in combination with other metal oxides in silicates. It commonly is found as sand in the vast ocean and river shores, their beds, deserts, rocks, and minerals. Silicon dioxide exists in several structural forms: polymorphic crystalline silica, synthetic quartz crystals, amorphous silica, and vitreous silica. This classification is not complete as there are other forms of silica synthesized for specialized applications. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Properties | white crystals or powder | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses | Functionalized RAFT agent for controlled radical polymerization; especially suited for the polymerization of styrene; acrylate and acrylamide monomers. Azide group can be used to conjugate to a variety of alkyne-functionalized biomolecules. Chain Transfer Agent (CTA). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses | SDS mixture of sodium alkyl sulfates consisting chiefly of sodium lauryl sulfate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Definition | ChEBI: A silicon oxide made up of linear triatomic molecules in which a silicon atom is covalently bonded to two oxygens. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses | manufacture of glass, water glass, refractories, abrasives, ceramics, enamels; decolorizing and purifying oils, petroleum products, etc.; in scouring- and grinding-compounds, ferrosilicon, molds for castings; as anticaking and defoaming agent. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard | Not toxic if ingested, inhaled silica dust can cause silicosis; carcinogen. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Safety Profile | The pure unaltered form is considered a nuisance dust. Some deposits contain small amounts of crystahne quartz and are therefore fibrogenic. When diatomaceous earth is calcined (with or without fluxing agents) some sdica is converted to cristobalite and is therefore fibrogenic. Tridymite has never been detected in calcined batomaceous earth. See also other silica entries | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purification Methods | Purification of silica for high technology applications uses isopiestic vapour distillation from concentrated volatile acids and is absorbed in high purity water. The impurities remain behind. Preliminary cleaning to remove surface contaminants uses dip etching in HF or a mixture of HCl, H2O2 and deionised water [Phelan & Powell Analyst 109 1299 1984]. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- SEASAND 7631-86-9 99%
- Inquiry
- 2024-11-05
-
VIP1年
- Mumal Microns Pvt Ltd
- 7631-86-9 98%
- Inquiry
- 2024-03-28
-
VIP1年
- Shri Vinayak Industries
- Silica 7631-86-9 98%
- Inquiry
- 2024-03-26
-
VIP1年
- HMP Minerals Pvt Ltd
- 7631-86-9 99%
- Inquiry
- 2024-03-23
-
VIP1年
- Shanti Chemical Works
- Silica precipitated 99%
- Inquiry
- 2024-03-16
-
VIP1年
- Star Earth Minerals Pvt Ltd
- 7631-86-9 Silica 98%
- Inquiry
- 2024-03-15
-
VIP1年
- KPS Chemicals And Pharmaceuticals
- Aerosil 98%
- Inquiry
- 2024-03-15
-
VIP1年
- Southern Petrochemical Industries Corporation (SPIC)
- 7631-86-9 Silica 98%
- Inquiry
- 2024-02-28
-
VIP1年
- Dr Khan Industrial Consultants Pvt Ltd
- Silicon dioxide 14808-60-7 / 14464-46-1 98%
- Inquiry
- 2024-02-21
-
VIP0年
- Chandra Chemical Industries
- 7631-86-9 99%
- Inquiry
- 2024-02-20
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
