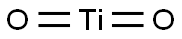| Chemical Properties |
The naturally occurring dioxide exists in three crystal forms: anatase, rutile and brookite. While rutile, the most common form, has an octahedral structure. Anatase and brookite have very distorted octahedra of oxygen atoms surrounding each titanium atom. In such distorted octahedral structures, two oxygen atoms are relatively closer to titanium than the other four oxygen atoms. Anatase is more stable than the rutile form by about 8 to 12 kJ/mol (Cotton, F.A., Wilkinson, G., Murillo, C.A and M Bochmann. 1999. Advanced Inorganic Chemistry, 6th ed, p. 697, New York: John Wiley & Sons) Other physical properties are: density 4.23g/cm3; Mohs hardness 5.8 g/cm3 ( anatase and brookite) and 6.2 g/cm3 ( rutile); index of refraction 2.488 (anatase), 2.583 (brookite) and 2.609 (rutile); melts at 1,843°C; insoluble in water and dilute acids; soluble in concentrated acids. |
| Uses |
Titanium dioxide is an extreme white and bright compound with high index of refraction. In paints it is a white pigment and an opacifying agent.It is in house paints, water paints, lacquers, enamels, paper filling and coating, rubber, plastics, printing ink, synthetic fabrics, floor coverings, and shoe whiteners. Also, it is used in colorants for ceramics and coatings for welding rods. A rutile form of the dioxide is used in synthetic gem stones. |
| Application |
| Industry |
Application |
Role/benefit |
| Pigment |
Optical coating for dielectric mirrors and gemstones |
Brightness and very high refractive index |
| Paper coating |
Helps to make paper whiter, brighter and more opaque |
| Plastics, adhesives and rubber |
Helps minimize the brittleness, fading and cracking that can occur as a result of light exposure |
| Food Contact materials and ingredients |
Prevents premature degradation and enhance the longevity of the product |
| Paints |
Gives paint its high gloss and rich depth of color |
| Ceramic glazes |
Acts as an opacifier and seeds crystal formation |
| Cosmetic |
Sunscreens |
Active ingredients/high refractive index and strong UV light absorbing capabilities |
| Daily cosmetics or make-up materials |
Additive/aids in hiding blemishes and brightening the skin |
| Toothpastes |
Additive/helps to whiten tooth |
| Catalyst |
Dye-sensitized solar cell |
Can produce electricity in nanoparticle form |
| Hydrolysis reaction |
Catalyzes the photo decomposition of water into hydrogen and oxygen |
| Automotive, power stations, etc. |
Helps to removes harmful exhaust gas emissions, such as nitrous oxides, volatile organic compounds, etc. |
| Detoxification or remediation of wastewater |
Photocatalytically mineralizes pollutants (to convert into CO2 and H2O) in waste water |
| Photocatalytic antimicrobial coating |
Photocatalytic destruction of organic matter |
| Others |
Oxygen sensor |
The electrical resistivity of TiO2 can be correlated to the oxygen content of the atmosphere |
| Anti-fogging coatings and self-cleaning windows |
Under exposure to UV light, TiO2 becomes increasingly hydrophilic |
| Coated ceramic tile |
Disinfectant and self-cleaning qualities |
| Treatment of the air in fruit, vegetable and cut flower storage areas |
Removes ethylene gas to prevent spoilage and prevents internal combustion |
| Memristor |
Can be employed for solar energy conversion |
| Mixed conductor |
Significant ionic and electronic conduction |
|
| Uses |
titanium dioxide (TiO2) is one of the 21 FDA-approved sunscreen chemicals with an approved usage level of 2 to 25 percent. When applied, titanium dioxide remains on the skin’s surface, scattering uV light. It is often used in conjunction with other sunscreen chemicals to boost the product’s SPF value, thus reducing the risk of irritation or allergies attributed to excessive usage of chemical sunscreens. Its incorporation into sunscreen formulations, makeup bases, and daytime moisturizers depends on the particular size of titanium dioxide employed. The smaller the particle size, the more unobtrusive Tio2’s application. Large particles, on the other hand, leave a whitish wash or look on the skin. Some companies list “micro” or “ultra” when referring to the size of the titanium dioxide particle. According to some sources, titanium dioxide could be the ideal uVA/uVB protection component given its chemical, cosmetic, and physical characteristics. Titanium dioxide is also used to provide a white color to cosmetic preparations. |
| Preparation |
Titanium dioxide is mined from natural deposits. It also is produced from other titanium minerals or prepared in the laboratory. Pigment-grade dioxide is produced from the minerals, rutile and ilmenite. Rutile is converted to pigment grade rutile by chlorination to give titanium tetrachloride, TiCl4. Anhydrous tetrachloride is converted back to purified rutile form by vapor phase oxidation.
Anatase form is obtained by hydrolytic precipitation of titanium(IV) sulfate on heating. The mineral ilmenite is treated with concentrated sulfuric acid. Heating the sulfate solution precipitates hydrous titanium oxide. The precipitate is calcined to expel all water.
Titanium dioxide also can be prepared by heating Ti metal in air or oxygen at elevated temperatures. |
| Uses |
Airfloated ilmenite is used for titanium pigment manufacture. Rutile sand is suitable for welding-rod-coating materials, as ceramic colorant, as source of titanium metal. As color in the food industry. Anatase titanium dioxide is used for welding-rod-coatings, acid resistant vitreous enamels, in specification paints, exterior white house paints, acetate rayon, white interior air-dry and baked enamels and lacquers, inks and plastics, for paper filling and coating, in water paints, tanners' leather finishes, shoe whiteners, and ceramics. High opacity and tinting values are claimed for rutile-like pigments. |
| Hazard |
Lower respiratory tract irritant. Possible carcinogen. |
| Safety Profile |
A nuisance dust. A human skin irritant. Questionable carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Violent or incandescent reaction with metals at high temperatures (e.g., aluminum, calcium, magnesium, potassium, sodium, zinc, lithium). See also TITANIUM COMPOUNDS. |