

2-Methyl-1-propanol
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product Name2-Methyl-1-propanol
- CAS No. 78-83-1
- EINECS No.201-148-0
- MFC4H10O
- MW74.12
- InChIKeyZXEKIIBDNHEJCQ-UHFFFAOYSA-N
- AppearanceSolidAPHA: ≤10
- Water Solubility 95 g/L (20 ºC)
- Boiling point 108 °C (lit.) 108 °C
- Melting point -108 °C (lit.)
- density 0.803 g/mL at 25 °C (lit.)
- storage temp. Store at +5°C to +30°C.
AD68
| 2-Methyl-1-propanol Basic information |
| Product Name: | 2-Methyl-1-propanol |
| Synonyms: | NATURAL ISO BUTYL ALCOHOL;2-METHYL-1-PROPANOL;2-Methyl propanol;2-METHYLPROPAN-1-OL;FEMA 2179;ISOBUTANOL;ISOBUTANOL, 2-METHYL-1-PROPANOL;ISOBUTYL ALCOHOL |
| CAS: | 78-83-1 |
| MF: | C4H10O |
| MW: | 74.12 |
| EINECS: | 201-148-0 |
| Product Categories: | Analytical Reagents;Analytical Reagents for General Use;Analytical/Chromatography;M-N;Plastic Bottles;Puriss p.a. ACS;Solvent Bottles;Solvent Packaging Options;Solvents;Amber Glass Bottles;CHROMASOLV for HPLC;Chromatography Reagents &;HPLC &;HPLC Grade Solvents (CHROMASOLV);HPLC/UHPLC Solvents (CHROMASOLV);Solvent by Application;UHPLC Solvents (CHROMASOLV);ACS and Reagent Grade Solvents;ACS Grade;ACS Grade Solvents;Carbon Steel Flex-Spout Cans;Semi-Bulk Solvents |
| Mol File: | 78-83-1.mol |
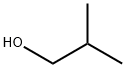 |
|
| 2-Methyl-1-propanol Chemical Properties |
| Melting point | −108 °C(lit.) |
| Boiling point | 108 °C(lit.) |
| density | 0.803 g/mL at 25 °C(lit.) |
| vapor density | 2.55 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| refractive index | n20/D 1.396(lit.) |
| FEMA | 2179 | ISOBUTYL ALCOHOL |
| Fp | 82 °F |
| storage temp. | Flammables area |
| solubility | water: miscible70g/L at 20°C |
| form | Solid |
| color | APHA: ≤10 |
| Relative polarity | 0.552 |
| PH | 7 (80g/l, H2O, 20℃) |
| explosive limit | 1.5-12%(V) |
| Water Solubility | 95 g/L (20 ºC) |
| Merck | 14,5131 |
| BRN | 1730878 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, aluminium. |
| CAS DataBase Reference | 78-83-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Propanol, 2-methyl-(78-83-1) |
| EPA Substance Registry System | 1-Propanol, 2-methyl-(78-83-1) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 10-37/38-41-67 |
| Safety Statements | 13-26-37/39-46-7/9 |
| RIDADR | UN 1212 3/PG 3 |
| WGK Germany | 1 |
| RTECS | NP9625000 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29051990 |
| Hazardous Substances Data | 78-83-1(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 2.46 g/kg (Smyth) |
| MSDS Information |
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| 2-Methyl-1-propanol Usage And Synthesis |
| Isobutanol | Isobutanol, also known as isopropyl alcohol, 2-methyl propanol is a colorless alcohol flammable liquid. Isobutanol is one of the main ingredients of fresh tea leaves, black tea and green tea to produce the wonderful aroma with the molecular weight of 74.12, boiling point of 107.66 ℃, relative density of 0.8016 (20/4 ℃), refractive index of 1.3959 and a flash point of 37 ℃. Isobutanol is fully dissolved in alcohol and ether, slightly soluble in water. Its vapor can form an explosive mixture with air; the explosion limit is 2.4% (volume). It can form addition compounds (CaCl2 • 3C4H10O) with calcium chloride. Isobutanol can be obtained by the distillation of the by-product of methanol and can also be derived from distillation of crude fusel oil. Using Industrial carbonyl cobalt as a catalyst, making propylene and carbon monoxide and hydrogen mixture react at 110~140 ° C, 2.0265 × 107~3.0397 × 107Pa to generate butyraldehyde and isobutyraldehyde, and then via catalytic hydrogenation, separation can obtain isobutanol. Isobutanol is used in the manufacture of petroleum additives, antioxidants, plasticizers, synthetic rubber, artificial musk, fruit oil and synthetic drugs and also used as solvents and chemical reagents. |
| Chemical properties | It is a kind of colorless transparent liquid with a special smell, soluble in water, and fully dissolved in ethanol and ether. |
| Uses | (1) For analysis reagents, chromatography reagents, solvents and extraction agent. (2) As raw materials for the organic synthesis, and also act as a superior solvent. (3) Isobutanol is raw materials for organic synthesis. It mainly used in the synthesis of isobutyronitrile, an intermediate for diazinon. (4) As raw materials of organic synthesis, isobutanol is used in the manufacture of petroleum additives, antioxidants, 2, 6-butylated hydroxytoluene, isobutyl acetate (paint solvents), plasticizers, synthetic rubber, artificial musk, fruit oil and synthetic drugs. It can also be used to purify strontium, barium and lithium salts and other chemical reagents and used as a superior solvent. (5) Extraction solvent. Food flavors listed in GB 2760-96. |
| Production method | (1) In the presence of sodium amalgam or other catalysts, it is derived from the reduction of butyraldehyde;r it is derived from the distillation of the by-product obtained from the synthesis of methanol. (2) 1. Carbonyl synthesis (by-product of butanol production from propylene) : using propylene and synthesis gas as raw material, going through carbonyl synthesis in the system to get n-butyl and isobutyl aldehyde, after the catalyst, hydrogenating into alcohol, then undergoing dehydration and separation can obtain the product of n-butyl and isobutyl alcohol respectively. 2. Hydrogenation of isobutyraldehyde: isobutyraldehyde via the liquid hydrogenation reaction, obtained isobutanol in the catalytic nickel. 3. Recycle from isobutyl oil, a byproduct of synthetic methanol distillation: isobutanol is obtained by dehydration of methanol, salting out and then azeotropic distillation of isobutyl oil. (3) The preparation method is using synthesis gas and propylene as raw materials, obtaining isobutyraldehyde during the carbonyl synthesis of 2-ethylhexyl alcohol, and then by hydrogenation to acquire isobutanol. |
| Hazards & Safety Information | Category: Flammable liquids Toxic classification: Moderately hazardous Acute toxicity: Oral-Rat LD50: 2460 mg/kg; Abdominal-Mouse LD50: 1801 mg/kg Stimulation data: Eye-Rabbit 2 mg Severity Explosives hazardous characteristics: To be explosive when mix with air. Flammability hazard characteristics: Flammable in case of fire, high temperature, oxidant; stimulating the smoke when combustion. Storage and transportation characteristics: Ventilation and low-temperature drying in treasury; oxidants and acids stored separately. Extinguishing agent: Dry powder, dry sand, carbon dioxide, foam. Occupational Standard: TWA 50 PPM. |
| Chemical Properties | colourless oily liquid |
| Uses | Isobutyl Alcohol is a reagent used in organic reactions. It is used in the synthesis of new fluorinating reagents. It is also used in the lipase-catalyzed production of biodiesel as an energy source. |
| Definition | ChEBI: An alkyl alcohol that is propan-1-ol substituted by a methyl group at position 2. |
| Uses | manufacture of esters for fruit flavoring essences; solvent in paint, varnish removers. |
| General Description | A clear colorless liquid with a sweet odor. Flash point 85 - 100°F. Less dense than water. Vapors heavier than air. |
| Air & Water Reactions | Highly flammable. Soluble in water. |
| Reactivity Profile | 2-Methyl-1-propanol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. 2-Methyl-1-propanol is incompatible with strong oxidizers. |
| Hazard | Flammable, moderate fire risk. Strong irritant. |
| Health Hazard | Contact with eyes is extremely irritating and may cause burns. Breathing vapors will be irritating to the nose and throat. In high concentrations, may cause nausea, dizziness, headache, and stupor. |
| Fire Hazard | HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
| Purification Methods | Isobutanol is dried by refluxing with CaO and BaO for several hours, followed by treatment with calcium or aluminium amalgam, then fractional distilling it from sulfanilic or tartaric acids. More exhaustive purifications involve formation of phthalate or borate esters. Heating it with phthalic anhydride gives the acid phthalate which, after crystallisation to constant melting point (m 65o) from pet ether, is hydrolysed with aqueous 15% KOH. The alcohol is distilled off as the water azeotrope and dried (K2CO3, then anhydrous CuSO4), and finally magnesium turnings, followed by fractional distillation. [Hückel & Ackermann J Prakt Chem 136 15 1933.] The borate ester is formed by heating the dried alcohol for 6hours in an autoclave at 160-175o with a quarter of its weight of boric acid. After fractional distillation under vacuum, the ester is hydrolysed by heating for a short time with aqueous alkali and the alcohol is dried with CaO and distilled. [Michael et al. J Am Chem Soc 38 653 1916.] Alternatively dry the alcohol with K2CO3, CaSO4 or CaCl2, filter and fractionally distil it. For further drying, the redistilled alcohol can be refluxed with the appropriate alkyl phthalate or succinate as described under ethanol. [Beilstein 1 IV 1588.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- 78-83-1 ISO-BUTYL ALCOHOL 99%
- Inquiry
- 2024-11-05
-
VIP1年
- SS Reagents and Chemicals
- 78-83-1 Isobutanol 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Pallav Chemicals And Solvents Pvt Ltd
- iso-Butanol 98.5% For Synthesis 78-83-1 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Bondbay Pharmaceuticals Pvt Ltd
- 78-83-1 98%
- Inquiry
- 2024-03-05
-
VIP1年
- Expresolv Limited
- 78-83-1 Isobutyl alcohol 98%
- Inquiry
- 2024-02-07
-
VIP1年
- The Andhra Petrochemicals Ltd. APL
- Isobutyl alcohol 78-83-1 98%
- Inquiry
- 2024-01-27
-
VIP1年
- Bharat Petroleum Corporation Limited
- Isobutyl alcohol 98%
- Inquiry
- 2024-01-19
-
VIP1年
- Ultra Chemical Works
- Iso Butanol 99%
- Inquiry
- 2024-01-02
-
VIP1年
- JSK Chemicals
- 78-83-1 Isobutanol, 99% 99%
- Inquiry
- 2023-12-22
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
