

Stearic acid
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameStearic acid
- CAS No.57-11-4
- EINECS No.266-928-5
- MFC18H36O2
- MW284.48
- InChIKeyQIQXTHQIDYTFRH-UHFFFAOYSA-N
- AppearancepowderWhite
- storage temp. Store below +30°C.
- Water Solubility 0.1-1 g/100 mL at 23 ºC
- Melting point 67-72 °C (lit.)
- Boiling point 361 °C (lit.)
- density 0.845 g/cm3
AD68
| Stearic acid Basic information |
| Product Name: | Stearic acid |
| Synonyms: | CETYLACETIC ACID;FEMA 3035;CARBOXYLIC ACID C18;C18;C18:0 FATTY ACID;ACIDUM STEARICUM 50;N-OCTADECYLIC ACID;N-OCTADECANOIC ACID |
| CAS: | 57-11-4 |
| MF: | C18H36O2 |
| MW: | 284.48 |
| EINECS: | 266-928-5 |
| Product Categories: | Miscellaneous Natural Products;Alkylcarboxylic Acids;Biochemistry;Higher Fatty Acids & Higher Alcohols;Monofunctional & alpha,omega-Bifunctional Alkanes;Monofunctional Alkanes;Saturated Higher Fatty Acids;Chemical intermediate;plasticizer, stabilizer and lubricant;Food additives |
| Mol File: | 57-11-4.mol |
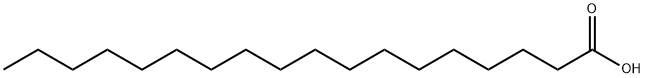 |
|
| Stearic acid Chemical Properties |
| Melting point | 67-72 °C(lit.) |
| Boiling point | 361 °C(lit.) |
| density | 0.84 |
| vapor pressure | 1 mm Hg ( 173.7 °C) |
| refractive index | 1.4299 |
| FEMA | 3035 | STEARIC ACID |
| Fp | >230 °F |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, soluble in ethanol (96 per cent) and in light petroleum (bp: 50-70 °C). |
| form | powder |
| pka | pKa 5.75±0.00(H2O t = 35) (Uncertain) |
| color | White |
| Water Solubility | 0.1-1 g/100 mL at 23 ºC |
| Merck | 14,8804 |
| BRN | 608585 |
| CAS DataBase Reference | 57-11-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Octadecanoic acid(57-11-4) |
| Safety Information |
| Hazard Codes | Xi,F |
| Risk Statements | 38-36/37/38-11 |
| Safety Statements | 37/39-26-16 |
| RIDADR | 1325 |
| WGK Germany | 3 |
| RTECS | WI2800000 |
| TSCA | Yes |
| HS Code | 38231100 |
| Hazardous Substances Data | 57-11-4(Hazardous Substances Data) |
| Toxicity | LD50 i.v. in mice, rats: 23±0.7, 21.5±1.8 mg/kg, L. Or, A. Wretlind, Acta Pharmacol. Toxicol. 18, 141 (1961) |
| MSDS Information |
| Provider | Language |
|---|---|
| Stearic acid | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Stearic acid Usage And Synthesis |
| description | Stearic acid is one of several major long-chain fatty acids comprising oils and fats. It is presented in animal fats, oil and some kinds of vegetable oils as wellin the form of glycerides. These oils, after hydrolysis, produce the stearic acid. Stearic acid is a fatty acid widely existing in nature and has the general chemical properties of carboxylic acids. Almost all kinds of fat and oil contain certain amount of stearic acid with the content in the animal fats being relative high. For example, the content in the butter can reach up to 24% while the content in vegetable oil is relative low with the value in tea oil being 0.8% and the oil in palm being 6%. However, the content in cocoa can reach as high as 34%. There are two major approaches for industrial production of stearic acid, namely fractionation and compression method. Add decomposition agent to the hydrogenated oil, and then hydrolyze to give the crude fatty acid, further go through washing with water, distillation, bleaching to obtain the finished products with glycerol as the byproduct. Most domestic manufacturers use animal fat for production. Some kinds of production technology will result in the incompletion of the distillation of fatty acid which produce stimulating odor at the time of the plastic processing and high temperatures. Although these odor is of no toxic but they will have certain effect on the working conditions and the natural environment. Most imported form of stearic acid takes vegetable oil as the raw materials, the production processes are more advanced; the produced stearic acid is of stable performance, good lubrication property and less odor in the application. Stearic acid is mainly used for the production of stearates such as sodium stearate, magnesium stearate, calcium stearate, lead stearate, aluminum stearate, cadmium stearate, iron stearate, and potassium stearate. The sodium or potassium salt of stearic acid is the component of soap. Although sodium stearate has a less decontamination ability than sodium palmitate, but its presence may increase the hardness of soap. Take butter as raw material, go through sulfuric acid or pressurized method for decomposition. The free fatty acids was first subject to water pressure method for removing the palmitic acid and oleic acid at 30~40 ℃, and then dissolved in ethanol, followed by addition of barium acetate or magnesium acetate which precipitates stearate. Then further add dilute sulfuric acid to get the free stearate acid, filter and take it, and re-crystallize in ethanol to obtain the pure stearic acid. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Chemical Properties | Pure product appears as white shiny soft small pieces. It is slightly soluble in water, soluble in alcohol, acetone, easily soluble in benzene, chloroform, ether, carbon tetrachloride, carbon disulfide, amyl acetate and toluene. |
| application | Stearic acid is widely used in cosmetics, plastics plasticizers, mold release agents, stabilizers, surfactants, rubber vulcanization accelerator, waterproof agent, polishing agent, metal soap, metal mineral flotation agents, softeners and pharmaceuticals as well as other organic chemicals. Stearic acid can also be used as the solvents of oil-soluble paint, crayons lubrication agent, stencil lighting agent and the emulsifier of stearic acid glyceride. Stearic acid can also be widely used in the manufacturing of PVC pipe, sheet material, profiles and film and is the PVC heat stabilizers with good lubricity and excellent stability against light and heat. In the application of polyvinyl chloride pipe, stearic acid helps prevent the "coke" during the processing and is effective heat stabilizer during PVC film processing while also preventing the discoloration of the finished film discoloration caused by exposure. Stearic acid has become the additive for lubrication, plasticization and stabilization of the filled masterbatch. Stearic acid can effectively improve the coating activating effect of inorganic powder and increase the flow rate of materials. When there is demand for a large flow rate of the melt for material with inorganic powder accounting for the most part, an appropriate increase in the content of stearic acid can significantly increase the melt flow rate of material. However, the amount of stearic acid used in filled masterbatch also have threshold with its amount being controlled in about 1% of the total mass. If the added amount is over-excessive, it will not only cause the decrease of the quality and the performance of plastic products but also generate sticky substance in the die lip location of the manufacturing equipment of the plastic products, affecting the production efficiency and product quality. The mono-or multi-alcohol ester of stearic acid can be used as cosmetics, nonionic surfactants and plasticizers. Its alkali metal salt can be dissolved in water and is a major component of soap. Other kinds of salts can be used as waterproofing agents, lubricants, bactericides, coating additives and PVC stabilizers. |
| Uses | It can be used as natural rubber, synthetic rubber (except butyl rubber) and latex curing active agent. It can also be used as raw material of plastic plasticizer and stabilizer. Medicine: it can be used for the preparation of ointments, suppositories, etc., as well as being used in the manufacture of cosmetics, candles, waterproof agent and polishing agent. The product can be used as a lubricant, defoamers and food additives in the food industry as well as the raw materials of glycerol stearate, stearic acid sorbitol anhydride esters and sucrose esters. It can also be used as standard reference product for gas analysis as well as the preparation of soap, cosmetics, pharmaceuticals and other organic chemicals. |
| Toxicity | Natural fatty acids; non-toxic. GRAS (FDA, §172.615, §184.1090, 2000). LD50: 21500mg/kg (rat via skin). |
| Limited use | FEMA (mg/kg): soft drinks: 2.0~ 10; candy: 4000; bakery: 3.5. GB 2760-2001: candy, gum base agent; take GMP as limit. |
| Production method | There are two major approaches for industrial production of stearic acid, namely fractionation and compression method. Add decomposition agent to the hydrogenated oil, and then hydrolyze to give the crude fatty acid, further go through washing with water, distillation, bleaching to obtain the finished products with glycerol as the byproduct. Compression method takes animal oil as raw material. Have animal oil subject to hydrolysis in the catalysis of zinc oxide at pressure of 1.17~1.47 MPa, further go through pickling, washing, distillation, cooling, freezing, press for removal of oleic acid to get the finished products. Heat the cotton seed oil, rice bran oil, or soybean oil in the presence of a hydrolyzing agent under normal pressure to boiling with hydrolysis of 1.5 h and harden to saturated fatty acid. Oleic acid hydrogenation; Use the C10~C20 and C18~C20 fraction of the synthetic fatty acid as raw materials, go through melting, pickling (with 1% sulfuric acid) mold, pressing, melting, pickling, dehydrating and crystallization to obtain it. It can be obtained through the low-temperature segment separation of the mixed fatty acid. It can also be made through the hydrogenation of oleic acid. |
| Chemical Properties | Stearic acid, CH3(CH2)16COOH, is a white or colorless, waxlike solid with a melting point of 70°C (158 OF), and a boiling point of 232°C (450 OF) at 2 kPa. It is soluble in alcohol, ether, and chloroform,and is insolublein water. Stearic acid, nature's most common fatty acid, is derived from natural animal and vegetable fats. Also known as n-octadecanoic acid, stearic acid is used in the preparation of metallic stearates, as a lubricant, and in pharmaceuticals, cosmetics, candles, and food packaging. |
| Uses | Pharmaceutic aid (emulsion adjunct); pharmaceutic aid (tablet and/or capsule lubricant). |
| Uses | stearic acid is an emulsifier and thickening agent found in many vegetable fats. Stearic acid is the main ingredient used in making bar soaps and lubricants. It occurs naturally in butter acids, tallow, cascarilla bark, and in other animal fats and oils. Stearic acid may cause allergic reactions in people with sensitive skin and is considered somewhat comedogenic. |
| Definition | ChEBI: A C18 straight-chain saturated fatty acid component of many animal and vegetable lipids. As well as in the diet, it is used in hardening soaps, softening plastics and in making cosmetics, candles and plastics. |
| Brand name | Hystrene 5016 (Witco). |
| General Description | White solid with a mild odor. Floats on water. |
| Air & Water Reactions | Slightly soluble in water. |
| Reactivity Profile | Stearic acid is incompatible with strong oxidizers and strong bases. Stearic acid is also incompatible with reducing agents. |
| Health Hazard | Compound is generally considered nontoxic. Inhalation of dust irritates nose and throat. Dust causes mild irritation of eyes. |
| Fire Hazard | Stearic acid is combustible. Stearic acid can heat spontaneously. |
| Safety Profile | Poison by intravenous route. A human sktn irritant. Questionable carcinogen with experimental tumorigenic data by implantation route. Combustible when exposed to heat or flame. Heats spontaneously. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Purification Methods | Crystallise stearic acid from acetone, acetonitrile, EtOH (5 times), aqueous MeOH, ethyl methyl ketone or pet ether (b 60-90o), or by fractional precipitation by dissolving in hot 95% EtOH and pouring into distilled water, with stirring. The precipitate, after washing with distilled water, is dried under vacuum over P2O5. It has also been purified by zone melting and partial freezing. [Tamai et al. J Phys Chem 91 541 1987, Beilstein 2 IV 1206.] |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
Recommended supplier
-
VIP1年
- AKASH PHARMA EXPORTS
- Stearic Acid 99%
- Inquiry
- 2024-05-16
-
VIP1年
- Om Minerals and Chemical
- Stearic acid 99%
- Inquiry
- 2024-03-28
-
VIP1年
- Shree Ashaka Chemicals
- 57-11-4 Stearic acid 99%
- Inquiry
- 2024-03-27
-
VIP1年
- HCC Group of Companies
- Stearic acid 99%
- Inquiry
- 2024-03-23
-
VIP1年
- Godrej Industries Ltd
- Stearic acid 99%
- Inquiry
- 2024-03-21
-
VIP1年
- Sudeep Pharma Pvt Ltd
- Stearic acid 98%
- Inquiry
- 2024-03-21
-
VIP1年
- Acme Synthetic Chemicals
- 57-11-4 Stearic acid 98%
- Inquiry
- 2024-03-20
-
VIP1年
- Arihant Innochem Pvt Ltd
- Stearic acid 57-11-4 99%
- Inquiry
- 2024-03-19
-
VIP1年
- Progress Life Sciences Pvt Ltd
- 57-11-4 Stearic acid 98%
- Inquiry
- 2024-03-04
-
VIP1年
- S V Plastochem Pvt Ltd
- Stearic acid 57-11-4 99%
- Inquiry
- 2024-02-14
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
